
Concept explainers
(a)
Interpretation:
The description of the molecule shown, having G as a generic substituent, is to be written.
Concept introduction:
An
Answer to Problem A.1P
The given molecule is a substituted ethane.
Explanation of Solution
The given molecule is:
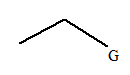
In this molecule, there are two carbon atoms in the straight chain. Hence, the alkane must be ethane
The given molecule is a substituted ethane.
(b)
Interpretation:
The description of the molecule shown, having G as a generic substituent, is to be written.
Concept introduction:
An alkane is said to be substituted if a hydrogen atom of the alkane is replaced by another atom or group of atoms. The atom or group of atoms which replaces the hydrogen atom is called a substituent. It is shown by G, which means a generic substituent.
Answer to Problem A.1P
The given molecule is a substituted pentane.
Explanation of Solution
The given molecule is:
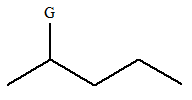
In this molecule, there are five carbon atoms in the straight chain. Hence, the alkane must be pentane
The given molecule is a substituted pentane.
Want to see more full solutions like this?
Chapter A Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- (a) What is the IUPAC name for the following molecule (including absolute configurations if required)? (b) Draw the Newman projections looking down the C3-C4 bond that represent all the three staggered conformations for the molecule shown above. (c) Given that the strain energies below, calculate the strain energies for each conformer and identify which is the most stable. Gauche interaction Strain Energy HOCH3 0.0 kJ mol- HOCH;CH; |0.4 kJ mol- 3.8 kJ mol- | 4.3 kJ mol- CH;CH3 CH;CH2CH3 |CH2CH; CH2CH; 4.6 kJ mol- Newman Projection of Conformer 1 Strain Energy of Conformer 1 Newman Projection of Conformer 2 Strain Energy of Conformer 2 Newman Projection of Conformer 3 Strain Energy of Conformer 3arrow_forwardDetergents need not be ionic. Pentaerythrityl palmitate (shown here) is a nonionic detergent used in dishwashingliquids.(a) Identify the hydrophilic and hydrophobic portions of the molecule.(b) Draw a depiction of a micelle that would form if this compound were dissolved in water.(c) What intermolecular interactions are primarily responsible for the micelle’s solubility in water?(d) What advantages do nonionic detergents have over ionic detergents in hard water?arrow_forwardWhich of the following is true about the carbon-carbon single bond in 1,3-butadiene when compared to the carbon-carbon single bond in ethane? (A) It is shorter than the carbon-carbon single bond of ethane. (B) The single bond in 1,3-butadiene has a higher %s character than in ethane The hydrogen atoms push the carbons closer using electrocyclic forces. Both A and B. (E) None of the above.arrow_forward
- Can you help me with the explanation of the alkane, alkene, alkyne, aromatic and all these terms give me examples too?arrow_forward[References] Give the name of the branched alkyl group attached to each of the following carbon chains, where the carbon chain is denoted by a horizontal line. Use the common names for C3-C4 alkyl groups, for example sec-butyl, etc. (a) (b) (c) (d) H₂ HC-C-CH3 CH₂ CH H3C CH3 CH₂ HC-CH3 HC-CH3 CH3 CH₂ CH₂ H₂C-CH3 H3C-CH₂H2-CH3 CH3 Submit Answer Try Another Version 6 item attempts remaining Previous Next>arrow_forwardPROBLEM Give IUPAC names for the following compounds. 4-28 (a) CH3 (b) H3C (c) н (e) H3C CH3 CH3 CH3 H (d) H Cara CH3 CH3 CH3arrow_forward
- Classify each ot the following structures as either an alcohol, carboxylic acid, or ester. Can you help me to explain to me that every part A though part F, please?arrow_forwardThe skeletal line formula for a branched alkene is shown below. (i) What is the molecular formula of this compound? (ii) How many carbon atoms are in the longest chain, ignoring the double bond? (iii) What is the longest chain incorporating both carbons of the double bond? (iv) How many substituents are on this chain? (v) Give the IUPAC name for this compound. [6]arrow_forwardDetermine the weakest C-H bond in each of the following compounds. (a) (b) (c) ОНarrow_forward
- PRACTICE PROBLEM 2.25 The compounds in each part below have the same (or similar) molecular weights. Which compound in each part would you expect to have the higher boiling point? Éxplain your answers. OH or HO. (a) (c) но OH or (b) (CHa),N orarrow_forward(iii) Draw the lowest energy conformer for the following compound.arrow_forwardPlease help , will provide helpful ratings for solving all 4 subparts only. Draw the skeletal structures that correspond to the following systematic (IUPAC) names.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





