
Osmotic pressure measurements can be used to determine the molecular weights of large molecules such as protein. For a solution of large molecule to qualify as “dilute,” its molar concentration must be very law and hence the osmotic pressure can be too small to measure accurately. For this reason, the usual procedure is to measure the osmotic pressure at a variety of concentrations, then extrapolate the results to the limit zero concentration. Here are some data,* for the protein hemoglobin dissolved in water at 3°C:
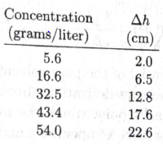
The quantity
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
An Introduction to Thermal Physics
Additional Science Textbook Solutions
Human Biology: Concepts and Current Issues (8th Edition)
College Physics: A Strategic Approach (3rd Edition)
Anatomy & Physiology (6th Edition)
Microbiology: An Introduction
Biology: Life on Earth with Physiology (11th Edition)
Organic Chemistry (8th Edition)
- A person is in a closed room (a racquetball court) with v=453 m3 hitting a ball (m 42.0 g) around at random without any pauses. The average kinetic energy of the ball is 2.30 J. (a) What is the average value of vx2 ? Does it matter which direction you take to be x ? (b) Applying the methods of this chapter, find the average pressure on the walls? (c) Aside from the presence of only one "molecule" in this problem, what is the main assumption in Pressure, Temperature, and RMS Speed that does not apply here?arrow_forwardIn the text, it was shown that N/V=2.681025m3 for gas at STP. (a) Show that this quantity is equivalent to N/V=2.681019cm3, as stated. (b) About how many atoms are mere in one m3 (a cubic micrometer) at STP? (c) What does your answer to part (b) imply about the separation of Mama and molecules?arrow_forwardA triple-point cell such as the one shown in Figure 19.20 (page 571) is constructed and calibrated at sea level. It is then shipped to Denver, which is 1 mi above sea level. Can the researchers in the Denver lab use the triple-point cell just as in a laboratory at sea level, or will special adjustments be needed? Explain.arrow_forward
- An ideal gas is trapped inside a tube of uniform cross-sectional area sealed at one end as shown in Figure P19.49. A column of mercury separates the gas from the outside. The tube can be turned in a vertical plane. In Figure P19.49A, the column of air in the tube has length L1, whereas in Figure P19.49B, the column of air has length L2. Find an expression (in terms of the parameters given) for the length L3 of the column of air in Figure P19.49C, when the tube is inclined at an angle with respect to the vertical. FIGURE P19.49arrow_forwardUnreasonable Results (a) How many moles per cubic meter of an ideal gas are there at a pressure of 1.001014N/m2 and at 0C ? (b) What is unreasonable about this result? (c) Which premise or assumption is responsible?arrow_forwardAn ideal gas is contained in a vessel at 300 K. The temperature of the gas is then increased to 900 K. (i) By what factor does the average kinetic energy of the molecules change, (a) a factor of 9, (b) a factor of 3, (c) a factor of 3, (d) a factor of 1, or (e) a factor of 13? Using the same choices as in part (i), by what factor does each of the following change: (ii) the rms molecular speed of the molecules, (iii) the average momentum change that one molecule undergoes in a collision with one particular wall, (iv) the rate of collisions of molecules with walls, and (v) the pressure of the gas?arrow_forward
- Consider again the box and particles with the speed distribution described in Problem 56. a. What is the average pressure exerted by the particles on the walls of the box? b. What is the average kinetic energy per particle in this box?arrow_forward(a) Given that air is 21% oxygen, find the minimum atmospheric pressure that gives a relatively safe partial pressure of oxygen of 0.16 atm. (b) What is the minimum pressure that gives a partial pressure of oxygen above the quickly fatal level of 0.06 atm? (c) The air pressure at the summit of Mount Everest (8848 m) is 0.334 atm. Why have a few people climbed it without oxygen, while some who have tried, even though they had trained at high elevation, had to tum back?arrow_forwardThe density of air in the Earths atmosphere decreases according to the function =0eh/h0, where 0=1.20kg/m3 is the density of air at sea level and h0 is the scale height of the atmosphere, with an average value of 7640 m. What is the maximum payload that a balloon filled with 2.50 103 m3 of helium (He=0.179kg/m3) can lift to an altitude of 10.0 km?arrow_forward
- The average translational kinetic energy for a molecule (Erans) is given by the following equation: Etrans =mv? Where m is the mass of the molecule and v? is the average of the square of the velocity. Given v2 = 3kT/m where k is the Boltzmann's constant, calculate the ratio of the kinetic energies at 200 °C and 100 °Carrow_forwardEstimate the fraction of molecular volume to the actual volume occupied by oxygen gas at STP. Take the diameter of an oxygen molecule to be 3 Åarrow_forwardHelium atoms have a mass of 4u and oxygen molecules have a mass of 32u, where u is defined as an atomic mass unit (u=1.660540×10−27 kg). Compare a gas of helium atoms to a gas of oxygen molecules. Part A: At what gas temperature TE would the average translational kinetic energy of a helium atom be equal to that of an oxygen molecule in a gas of temperature 300 K? Part B: At what gas temperature Trms would the root-mean-square (rms) speed of a helium atom be equal to that of an oxygen molecule in a gas at 300 K?arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College




