
Concept explainers
Interpretation:
Non-polar and polar side of amino acid needs to be explained.
Concept introduction:
Proteins are consist of amino acids. Each amino acid has at least one
The presence of the alkyl group generated the non-polar property for amino acid, while the polar side chains are present in the form of a functional group.
Answer to Problem 6A
Non-polar side chains in alpha amino acid tend to be cluster together, whereas polar side chains are most often hydrophilic in an aqueous medium.
Explanation of Solution
The amino acid is represented as −
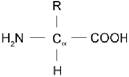
Where the R group act as a non-polar side and amine, carboxylic groups are polar in nature.
The non-polar group is hydrophobic in nature and the functional group shows the polar character.
The functional groups present in amino acids termed as polar groups and the side chains as alkyl groups are termed as non-polar groups.
The hydrophilic and hydrophobic properties of the amino acid represented by the different functional groups and side chains attached to the central carbon of amino acid.
Chapter 21 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





