
Concept explainers
Interpretation: The lock and key model needs to be matched with the correct option.
Concept Introduction:
Enzyme is composed of proteins. This is a substance that performed as catalyst and maintaining the
They act on substrate and formed a complex that is enzyme substrate complex and converted into product.
Answer to Problem 3STP
Correct answer: Option (C) the function of the enzymes.
Explanation of Solution
Reason for correct option:
The lock and key model applies for the function of the enzymes, where the enzyme acted as key and substrate is the lock. The interaction of enzyme and substrate is follows the lock and key theory. The interactions of a particular site with the enzyme is called active center. The enzyme substrate complex is formed the product.
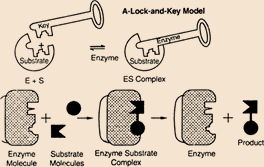
Reasons for incorrect options:
Option (A) the structure of carbohydrates in not explain by the lock and key model, hence it is incorrect option.
Option (B) the shape of the proteins is not related to the lock and key model. So it is incorrect for the lock and key model.
Option (D) glycoside linkage is a type of bonding is not explain by the lock and key model. Hence it is incorrect option.
Chapter 21 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





