
Concept explainers
Interpretation:
The secondary structure of proteins needs to be explained.
Concept introduction:
Protein − proteins are composed of the different types of amino acids and they are bonded together with the help of the peptide bonds.
There are four general classes are known for the proteins. The arrangements of the amino acid define the structure of the proteins.
Answer to Problem 11A
The complex structure of the proteins is known as a secondary structure. The amino acid interacts with each other by the formation of a peptide bond.
Explanation of Solution
The common secondary structure of proteins is consists of the beta-pleated sheet. In this structure, two different amino acid chains interact with the other one. The linkage is a polypeptide. The chains of the amino acid are bound by hydrogen bonds. The two types of beta-pleated sheets are parallel with other beta-pleated sheets and opposite with the beta-pleated sheets and form a complex secondary structure of proteins.
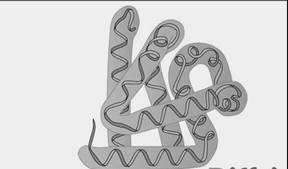
Secondary structure of protein
The coiled helical and pleated structure is representing the secondary structure of proteins. This structure is made up of two antiparallel strands. The secondary structure of proteins is composed of hydrogen bonds formed between atoms of the polypeptide backbone.
Chapter 21 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





