
Concept explainers
Interpretation:
The general formula of an alpha-amino acid and common structure in all alpha-amino acid needs to be determined.
Concept introduction:
Proteins are macromolecule they are composed of the amino acid. These amino acids are attached linearly to form long linear chains called polypeptides, which create a specific three-dimensional shape. Proteins have different shapes according to there amino acid sequence.
The amino acid is consist of
Answer to Problem 5A
The general structure for an alpha-amino acid is given as-
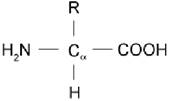
The general structure of an alpha-amino acid is composed of an alpha carbon at the central portion and at least one carboxylic group and one amine group and attached side chain.
Explanation of Solution
The general structure of an alpha-amino acid is given as -
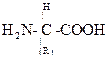
The central carbon is representing the alpha carbon and one amine group, carboxylic group and R is the side chain.
In all alpha-amino acids the common structure is alpha carbon attached with the amine, carboxylic group, and side-chain R varies.
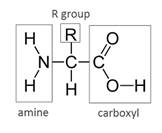
The central carbon is alpha carbon attached to four different groups. The common structure in all alpha-amino acids is central carbon and amine, carboxylic group.
Chapter 21 Solutions
World of Chemistry, 3rd edition
- Differentiate between single links and multicenter links.arrow_forwardI need help on my practice final, if you could explain how to solve this that would be extremely helpful for my final thursday. Please dumb it down chemistry is not my strong suit. If you could offer strategies as well to make my life easier that would be beneficialarrow_forwardNonearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





