
Concept explainers
Interpretation:
The double-helix structure of DNA, and types of bonding needs to be explained.
Concept introduction:
DNA- deoxyribonucleic acid, it is a genetic material for a cell. Mainly it is found in the nucleus of the cell. It is consisting of a deoxyribose sugar, a phosphate, and nitrogenous bases.
Answer to Problem 34A
The double helix structure of DNA is a complex and twisted ladder-like structure where the deoxy sugar, nitrogenous base, and phosphate group are present.
There are two types of bonding present in the DNA, one is covalent and the other one is hydrogen bonding.
Explanation of Solution
The most acceptable structure of DNA is the Double helix structure. It gives detailed information on the structure of a DNA molecule. There are two strands in a DNA molecule which are twisted with each other and representing a twisted ladder. DNA single-strand has a backbone made of deoxyribose and phosphate groups. The nitrogenous bases in DNA are adenine (A), guanine (G), thymine (T), and cytosine (C).
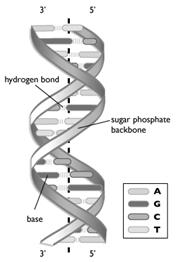
The nitrogenous base is interconnected with hydrogen bonding, and sugar and phosphate groups structure are bonded with a covalent bond.
The DNA double helix
The nitrogenous bases of two strands are linked with hydrogen bond and the covalent type of bonding with the complementary base pairing occurs within the chain of each strand of the double helix.
Chapter 21 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





