
Concept explainers
Interpretation:The structures of six different tripeptide possible from phenylalanine, alanine and glycine (each taken only once) needs to be drawn. The peptide bonds needs to be circled. The terminal amino and carboxyl groups need to be labelled.
Concept Introduction:Proteins are
These amino acids are involved in condensation process to form peptides and polypeptides, which further form complex protein molecules. Amino acids are the organic molecules with both
Answer to Problem 10A
- Phenylalanine-alanine -glycine
- Phenylalanine-glycine-alanine
- Alanine-glycine-phenylalanine
- Alanine-phenylalanine-glycine
- Glycine-alanine-phenylalanine
- Glycine-phenylalanine-alanine
Explanation of Solution
In a tripeptide, N-terminus must be written on left side whereas the C-terminus must be on right side. There are 6 possible combinations with phenylalanine, alanine and glycine are:
- Phenylalanine-alanine -glycine
- Phenylalanine-glycine-alanine
- Alanine-glycine-phenylalanine
- Alanine-phenylalanine-glycine
- Glycine-alanine-phenylalanine
- Glycine-phenylalanine-alanine
The structure of tripeptide phenylalanine-alanine -glycine is as follows:
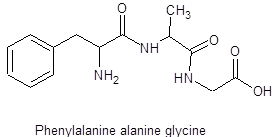
Here, peptide bonds, terminal amino and carboxylic groups are labelled as follows:
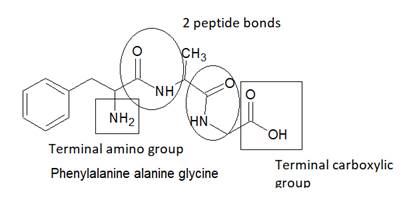
The structure of tripeptide phenylalanine-alanine -glycine is as follows:
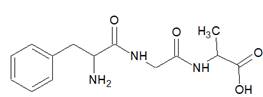
Phenylalanine-glycine-alanine
Here, peptide bonds, terminal amino and carboxylic groups are labelled as follows:
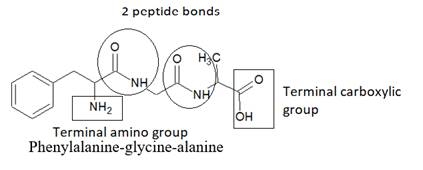
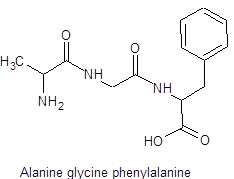
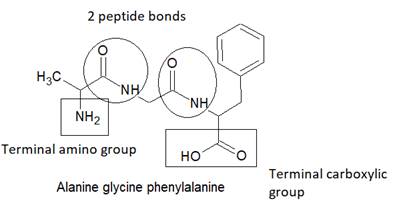
The structure of tripeptide Alanine-glycine-phenylalanine is as follows:
Here, peptide bonds, terminal amino and terminal carboxylic group are represented as follows:
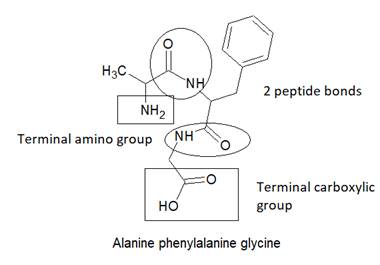
The structure of alanine-phenylalanine-glycine is as follows:
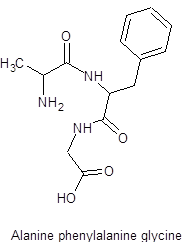
Here, peptide bonds, terminal amino and terminal carboxylic group are represented as follows:
The structure of Glycine- alanine- phenylalanine is as follows:
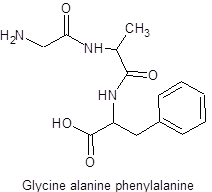
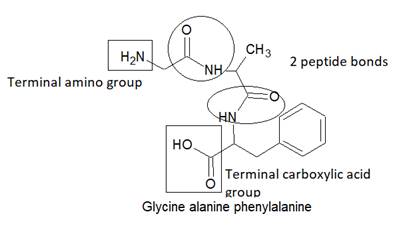
Here, peptide bonds, terminal amino and terminal carboxylic group are represented as follows:
The structure of Glycine-phenylalanine-alanine is as follows:
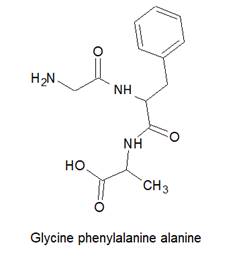
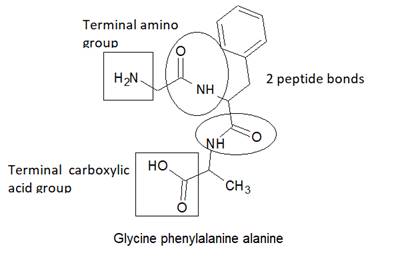
Here, peptide bonds, terminal amino and terminal carboxylic group are represented as follows:
Thus, three amino acids can form 6 possible combinations.
Chapter 21 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





