
Concept explainers
(a)
Interpretation:
The reaction and the alcohol that is used for the preparation of ethanal have to be given.
Concept Introduction:
The oxidation of a primary alcohol produces an aldehyde.
The generalized equation is written as,
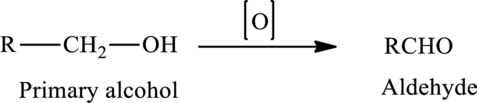
The oxidation of a secondary alcohol produces a ketone.
The generalized equation is written as,
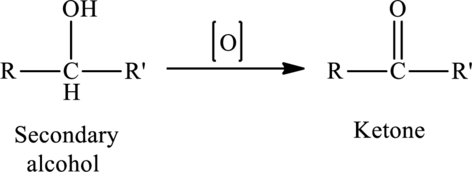
(b)
Interpretation:
The reaction and the alcohol that is used for the preparation of 2-octanone have to be given.
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The reaction and the alcohol that is used for the preparation of 5-methyloctanal have to be given.
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
Chemical Principles: The Quest for Insight
- Draw the organic product formed when the following compounds undergo a substitution reaction:(a) acetic and 1-hexanol; (b) propanoic acid and dimethylamine; (c) ethanoic acid and dimethylamine.arrow_forwardGive the structural formulae and name the functional groups of the following compounds. (a) 3-chlorobut-1-ene Name the functional group: (b) butanedioic acid Name the functional group: (c) propanamide Name the functional group: (d) 3-methylbutanal Name the functional group:arrow_forward5) Provide complete and balanced chemical reactions for the following. Write the formula and the name of the alcohol that when dehydrated, gives rise to the following alkenes. Give complete reactions. (а) СH-CH,CH-CH-CHCH-CHa (b) • 3-ethyl-1-hexanol is a primary alcohol. Give the two complete oxidation reactions using potassium permanganate.arrow_forward
- What is the structure of the alcohol produced when 3-methyl-1-pentene undergoes (a) acid catalyzed hydration (b) oxymercuration/demercuration (c) hydroboration/oxidationarrow_forward5.Write the structural formula of the ester that, when hydrolyzed, would yield the following:(a) methanol and propanoic acid(b) 1-octanol and acetic acid (c) ethanol and butanoic acidarrow_forwardUsing condensed structural formulas, write a balancedchemical equation for each of the following reactions:(a) hydrogenation of cyclohexene, (b) addition of H2O totrans-2-pentene using H2SO4 as a catalyst (two products),(c) reaction of 2-chloropropane with benzene in the presenceof AlCl3.arrow_forward
- (i) Draw the structural formula of compounds L, M, N and P (ii) Name the type of chemical reaction for the formation of compound N.arrow_forward(1) Write a complete chemical equation showing reactants, products, and catalysts needed (if any) for the following reaction and (2) Draw and name the organic compound found in every reaction. (a) Complete hydrogenation of 2-Methylhexa-1,5-diene (b) Complete halogenation (Br2) of 3-Ethyl-2,2-dimethylhept-3-ene (c) Reaction of (4E)-2.4-Dimethylhexa-1,4-diene with a mole of water (d) Reaction of cis-3,3-Dimethyl-4-propylocta-1,5-diene with two mole of HBr (e) Reaction of trans-1-Bromo-3-chlorocyclopentane with potassium hydroxide (f) Formation of Gilman reagent using isopropyl bromide (g) Ozonolysis of 3,3-Dimethyloct-4-yne (h) Complete halogenation (Cl2) of 3-Ethyl-5-methyl-1,6,8-decatriyne (i) Partial hydrogenation using Lindlar's Catalyst 2,2,5,5-Tetramethylhex-3-yne (i) Reaction of 3.4-Dimethylcyclodecyne with sodium amidearrow_forward2. Write the structure for the most oxidized organic product when each of the following is reacted with potassium dichro- mate. Write NR if no reaction occurs.(a) methanol (b) ethyl alcohol (c) 2,3,4-trimethyl-2-pentanol (d) 3-hexanol 3. Alcohols can be made by reacting alkyl halides with sodium hydroxide as follows: RX +. NaOH ROH + NaX Give the names and formulas of the alcohols produced from the following alkyl bromides by this method:(a) 2-bromobutane(b) 2-bromo-3-ethylpentane (c) bromocyclopentanearrow_forward
- (a) Write a chemical test to distinguish between: (i) Chlorobenzene and Benzyl chloride. (ii) Chloroform and Carbon tetrachloride. (b) Why is methyl chloride hydrolysed more easily than chlorobenzene?arrow_forwardAcetyl chloride, CH3COCl, reacts with the hydroxyl groupsof alcohols to form ester groups with the elimination ofHCl. When an unknown compound X with formulaC4H8O3 reacted with acetyl chloride, a new compound Ywith formula C8H12O5 was formed.(a) How many hydroxyl groups were there in X?(b) Assume that X is an aldehyde. Write a possible structure for X and a possible structure for Y consistent with your structure for X.arrow_forwardDraw the structure and name the product formed if the following alcohols are oxidized. Assume an excess of the oxidizing agent is used. If the alcohol is not expected to react with a chemical oxidizing agent, write NR (no reaction).(a) CH3CH2CH2CH2OH(b) 2-butanol(c) 2-methyl-2-propanol(d) 2-methyl-1-propanolarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





