
Concept explainers
Compound C
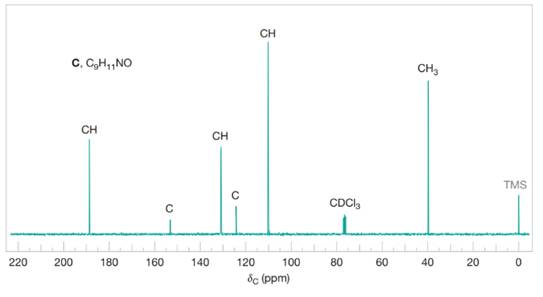
Figure 5 The broadband proton-decoupled
Want to see the full answer?
Check out a sample textbook solution
Chapter SRP Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: Matter and Change
Chemistry & Chemical Reactivity
Chemistry: A Molecular Approach
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: The Central Science (13th Edition)
- Treatment of alcohol A (molecular formula C5H12O) with CrO3, H2SO4, and H2O affords B with molecular formula C5H10O, which gives an IR absorption at 1718 cm−1. The 1H NMR spectrum of B contains the following signals: 1.10 (doublet, 6 H), 2.14 (singlet, 3 H), and 2.58 (septet, 1 H) ppm. What are the structures of A and B?arrow_forwardAcid-catalyzed hydrolysis of HOCH2CH2C(CH3)2CN forms compound A (C6H10O2). A shows a strong peak in its IR spectrum at 1770 cm-1 and the following signals in its 1H NMR spectrum: 1.27 (singlet, 6 H), 2.12 (triplet, 2 H), and 4.26 (triplet, 2 H) ppm. Draw the structure for A and give a stepwise mechanism that accounts for its formation.arrow_forwardDeduce the identity of the following compound from the spectral data given. C8H10: 1H NMR, 6 1.20 (3H, triplet), 2.60 (2H, quartet), 7.12 (5H, singlet) (ppm); IR, 3050, 2970, 1600 cm-1; MS, m/z 91arrow_forward
- Reaction of (CH3)3CCHO with (C6H5)3P=C(CH3)OCH3, followed bytreatment with aqueous acid, affords R (C7H14O). R has a strong absorption in its IR spectrum at 1717 cm−1 and three singlets in its 1H NMR spectrum at 1.02 (9 H), 2.13 (3 H), and 2.33 (2 H) ppm. What is thestructure of R?arrow_forwardAn unknown compound has a molecular formula of C,H,O. Its IR spectrum shows prominent absorptions at 2980, 2960, and 1718 cm . It exhibits the following signals in its H NMR spectrum (ppm): 1.06 (triplet, 3H), 2.12 (singlet, 3H), 2.45 (quartet, 2H); and the following signals in its ¹3C NMR spectrum ( ppm): 7.6, 29.5, 36.8, 208.8. Draw the structure of the unknown compound. Click and drag to start drawing a structure. 0 X 0:0arrow_forwardA hydrocarbon, compound B, has molecular formula C6H6, and gave an NMR spectrum with two signals: delta 6.55 pm and delta 3.84 pm with peak ratio of 2:1. When warmed in pyridine for three hr, compound B quantitatively converts to benzene. Mild hydrogenation of B yielded another compound C with mass spectrum of m/z 82. Infrared spectrum showed no double bonds; NMR spectrum showed one broad peak at delta 2.34 ppm. With this information, address the following questions. a) How many rings are in compound C? b) How many rings are probably in B? How many double bonds are in B? c) Can you suggest a structure for compounds B and C? d) In the NMR spectrum of B, the up-field signal was a quintet, and the down field signal was a triplet. How must you account for these splitting patterns?arrow_forward
- Compound A is treated with a mixture of nitric and sulfuric acids to generate Compound B. The 1H-NMR spectrum of B shows two singlets, one at 2.52 pm and one at 8.13 ppm. The 13C-NMR spectrum of B shows five signals. The mass spectrum of B shows a peak at m/z = 260 and another peak at m/z = 262; the relative height of the two peaks is 1:1 respectively. - Identify compound B, explaining your reasoningarrow_forwardThymol (molecular formula C10H14O) is the major component of the oil ofthyme. Thymol shows IR absorptions at 3500–3200, 3150–2850, 1621, and1585 cm−1. The 1H NMR spectrum of thymol is given below. Propose apossible structure for thymol.arrow_forwardThere are several isomeric alcohols and ethers of molecular formula C5H12O. Two of these exhibit the following 1H-NMR spectra. Propose a structure for each of the isomers. Isomer A: δ = 0.92 (t, 7.8 Hz, 3 H), 1.20 (s, 6H), 1.49 (q, 7.8 Hz, 2H), 1.85 (s, 1H) ppm Isomer B: δ = 1.19 (s, 9 H), 3.21 (s, 3H) ppmarrow_forward
- Compound A is a hydrocarbon with a molar mass of 96g/mol, with the given C13 spectral data. When compound A reacts with BH3 followed by the treatment with basic H2O2 it is converted to compound B. Propose structures for A and B, explain your analysis.Compound A- Proton decoupled C NMR: 26.8, 28.7, 35.7, 106.9, 149.7 δ.DEPT-90: No peak.DEPT-135: No positive peaks; negative peaks at 26.8, 28.7, 35.7, 106.9 δ.Compound B- Proton decoupled C NMR: 26.1, 26.9, 29.9, 40.5, 68.2 δ.DEPT-90: 40.5 δ.DEPT-135: positive peak at 40.5 δ; negative peaks at 26.1, 26.9, 29.9, 68.2 δarrow_forwardThese are the 1H RMN and IR spectrums of an A compound with formula C10H13NO2. When A reacts with NaOH in water and heat, B compound is formed, with formula C10H11NO. What are the structures of A and B?arrow_forwardThe 'H NMR spectrum of compound A (C3H100) has four signals: a multiplet at 8 = 7.25-7.32 ppm (5 H), a singlet at d = 5.17 ppm (1 H), a quartet at d = 4.98 ppm (1 H), and a doublet at ô = 1.49 ppm (3 H). There are 6 signals in its 13C NMR spectrum. The IR spectrum has a broad absorption in the -3200 cm-1 region. Compound A reacts with KMNO4 in a basic solution followed by acidification to give compound B with the molecular formula C7H6O2. Draw structures for compounds A and B.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
