
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter SRP, Problem 27P
Interpretation Introduction
Interpretation:
The structure of the intermediate compound from A to M is to be written and the intermediate formed by the reaction of compound D with N-Bromo succinimide, the stereoisomer of Dianeackerone, are to be represented.
Concept introduction:
Dianeackerone consists of nineteen atoms of carbon, thirty atoms of hydrogen and one tom of oxygen due to which its molecular formula is 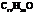 . The IUPAC name of this compound is
. The IUPAC name of this compound is 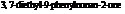 . It is a natural product and has a volatile behavior. It has both
. It is a natural product and has a volatile behavior. It has both 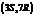 and
and 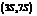 stereoisomers.
stereoisomers.
N-Bromo succinimide, also known as NBS, is a type of brominating agent, which is used as a source of bromine in many reactions.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Compounds A and B are isomers having the molecular formula C4H8O3. Identify A and B on the basis of their 1H NMR spectra.Compound A: δ 1.3 (3H, triplet); 3.6 (2H, quartet); 4.1 (2H, singlet); 11.1 (1H, broad singlet)Compound B: δ 2.6 (2H, triplet); 3.4 (3H, singlet); 3.7 (2H triplet); 11.3 (1H, broad singlet)
Furan undergoes electrophilic aromatic substitution more readily than benzene; mild reagents and conditions are sufficient.For example, furan reacts with bromine to give 2-bromofuran.(a) Propose mechanisms for the bromination of furan at the 2-position and at the 3-position. Draw the resonance forms ofeach sigma complex, and compare their stabilities.(b) Explain why furan undergoes bromination (and other electrophilic aromatic substitutions) primarily at the 2-position.O Br O123furan 2-bromofuran
Treatment of compound E (molecular formula C4H8O2) with excessCH3CH2MgBr yields compound F (molecular formula C6H14O) afterprotonation with H2O. E shows a strong absorption in its IR spectrum at1743 cm−1. F shows a strong IR absorption at 3600−3200 cm−1. The 1HNMR spectral data of E and F are given. What are the structures of E andF?Compound E signals at 1.2 (triplet, 3 H), 2.0 (singlet, 3 H), and 4.1 (quartet, 2 H) ppm Compound F signals at 0.9 (triplet, 6 H), 1.1 (singlet, 3 H), 1.5 (quartet, 4H), and 1.55 (singlet, 1 H) ppm
Chapter SRP Solutions
Organic Chemistry
Ch. SRP - 1. Arrange the compounds of each of the following...Ch. SRP - 2. Arrange the compounds of each of the following...Ch. SRP - Predict the final product from each of the...Ch. SRP - Prob. 4PCh. SRP - Write detailed mechanisms for each of the...Ch. SRP - Prob. 6PCh. SRP - Prob. 7PCh. SRP - Prob. 8PCh. SRP - Prob. 9PCh. SRP - Give stereochemical structures for compounds AD:
Ch. SRP - Prob. 11PCh. SRP - The remaining steps in the industrial synthesis of...Ch. SRP - Prob. 13PCh. SRP - Prob. 14PCh. SRP - Prob. 15PCh. SRP - Prob. 16PCh. SRP - 17. Show how you would modify the synthesis given...Ch. SRP - Prob. 18PCh. SRP - Give structures for compounds AD. Compound D gives...Ch. SRP - The tranquilizing drug meprobamate (Equanil or...Ch. SRP - Prob. 21PCh. SRP - 22. Outlined here is the synthesis of a central...Ch. SRP - 23. What are compounds A and B? Compound B has a...Ch. SRP - Prob. 24PCh. SRP - 25. The Dow process for synthesizing phenol, which...Ch. SRP - Prob. 26PCh. SRP - Prob. 27PCh. SRP - 28. Compound Y shows prominent IR absorption...Ch. SRP - Prob. 29PCh. SRP - Consider this reaction involving peracetic acid:...Ch. SRP - 31. A compound (N) with the molecular formula...Ch. SRP - 32. Compound X is insoluble in aqueous sodium...Ch. SRP - Write the structures of the three products...Ch. SRP - Compound C (C9H11NO) gives a positive Tollens test...Ch. SRP - A compound X (C10H14O) dissolves in aqueous sodium...Ch. SRP - Compound Z (C5H10O) decolorizes bromine. The IR...Ch. SRP - 37. Compound W was isolated from a marine annelid...Ch. SRP - 38. Phenols generally are not changed on treatment...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Treatment of 1,3,6-cyclononatriene (Compound 1), or its dimethyl derivative (Compound 2), with potassium amide (KNH₂) in liquid ammonia results in the formation of anion 1a or 2a, respectively (J. Am. Chem. Soc. 1973, 95, 3437-3438): 9 15.85a 2 6 3 4 5 Compound 1 (R=H) Compound 2 (R=CH3) * You answer is incorrect. KNH₂ KNH₂ 1a (anion) 2a (anion) Of the following, which is NOT one of the four resonance structures of 1a?arrow_forwardThere are several isomeric alcohols and ethers of molecular formula C5H12O. Two of these exhibit the following 1H-NMR spectra. Propose a structure for each of the isomers. Isomer A: δ = 0.92 (t, 7.8 Hz, 3 H), 1.20 (s, 6H), 1.49 (q, 7.8 Hz, 2H), 1.85 (s, 1H) ppm Isomer B: δ = 1.19 (s, 9 H), 3.21 (s, 3H) ppmarrow_forwardAs reaction of (CH3)2CO with LIC≡CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600−3200, 3303, 2938, and 2120 cm−1. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?arrow_forward
- Three compounds, A, B, and C, have the same molecular formula, C5H6. In the presence of a platinum catalyst, all three compounds absorb 3 molar equivalents of hydrogen and yield pentane. Compounds B and C give a precipitate when treated with ammoniacal silver nitrate; compound A give no reaction. Compounds A and B show an absorption maximum near 250 nm. Compound C shows no absorption maximum beyond 200 nm. Propose a structure for A, B, and C.arrow_forwardCompound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forwardAs we will learn in Chapter 17, reaction of (CH3)2CO with LIC≡CH followed by H2O affords compound D, which has a molecular ion in its mass spectrum at 84 and prominent absorptions in its IR spectrum at 3600−3200, 3303, 2938, and 2120 cm−1. D shows the following 1H NMR spectral data: 1.53 (singlet, 6 H), 2.37 (singlet, 1 H), and 2.43 (singlet, 1 H) ppm. What is the structure of D?arrow_forward
- Compound A has molecular formula C5H8Br4 but shows only one singlet in the 1H-NMR spectrum. Suggest a structure for A and explain your reasoning.arrow_forwardCompound A has molecular formula C5H10O. It shows three signals in the 1H-NMR spectrum - a doublet of integral 6 at 1.1 ppm, a singlet of integral 3 at 2.14 ppm, and a quintet of integral 1 at 2.58 ppm. Suggest a structure for A and explain your reasoning.arrow_forwardCompounds B and C are isomers with molecular formula C5H9BrO2. The 1H NMR spectrum of compounds B and C are shown below. The IR spectrum corresponding to compound B showed strong absorption bands at 1739, 1225, and 1158 cm-1, while the spectrum corresponding to compound C have strong bands at 1735, 1237, and 1182 cm-1. 1.Based on the information provided, determine the structure of compounds B and C. 2.Assign all peaks in 1H NMR spectrum of compounds B and C.arrow_forward
- Treatment of 2-methylpropanenitrile [(CH3)2CHCN] with CH3CH2CH2MgBr, followed by aqueous acid, affords compound V, which has molecular formula C7H14O. V has a strong absorption in its IR spectrum at 1713 cm−1, and gives the following 1H NMR data: 0.91 (triplet, 3 H), 1.09 (doublet, 6 H), 1.6 (multiplet, 2 H), 2.43 (triplet, 2 H), and 2.60 (septet, 1 H) ppm. What is the structure of V? We will learn about this reaction in Chapter 20.arrow_forwardTreatment of compound C (molecular formula C4H8O) with C6H5MgBr, followed by H2O, affords compound D (molecular formula C10H14O). Compound D has a strong peak in its IR spectrum at 3600–3200 cm−1. The 1H NMR spectral data of C and D are given. What are the structures of C and D? Compound C signals at 1.3 (singlet, 6 H) and 2.4 (singlet, 2 H) ppm Compound D signals at 1.2 (singlet, 6 H), 1.6 (singlet, 1 H), 2.7 (singlet, 2 H), and 7.2 (multiplet, 5 H) ppmarrow_forwardTreatment of ketone A with ethynyllithium (HC≡CLi) followed by D3O+ afforded a compound B of molecular formula C12H13DO3, which gave an IR absorption at approximately 1715 cm−1. What is the structure of B and how is it formed?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning