
Concept explainers
Consider this reaction involving peracetic acid:
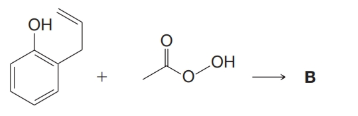
These are spectral data for the product, B:
(b) Propose a mechanism for formation of B.
Want to see the full answer?
Check out a sample textbook solution
Chapter SRP Solutions
Organic Chemistry
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
Basic Chemistry (5th Edition)
Chemistry: A Molecular Approach (4th Edition)
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
CHEMISTRY-TEXT
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
- Did you first add the H2O(l) on the right side of the equaton and then added the 2HO- on the left side and multiplied CIO2 on the left side by 2?arrow_forwardWhen reducing 4-nitrobenzaldehyde to 4-nitrobenzyl alcohol, the reducing agent of choice is LIAIH4 (lithium aluminum hydride) because it reduces the nitro group to an amine. O 1) True O 2) Falsearrow_forwardGive each product in the series of reactions shown below CH;CI HNO3 А OH CUCN E Sn NaNO2, HCI. D ►F AICI3 H2SO4 HCI 0°Carrow_forward
- Consider the pair of reactions below to answer the following questions. 70% 30% CH₂OH OH (A) (B) 50% DMF 50% acetone Y' 8. Which reaction is slower A or B хон + HCI + HCI bBarrow_forward1. 1a) 1b) 1c) 1d) For each of the following reactions: Draw a reaction mechanism (curved arrows) and comeplete the reaction on the right side. Then predict which side of the equilibrium is favored, left, right or either. Circle the stronger acid. If the strength are euqal, circle both. Give one word from the HIRE rules to state the most important factor in your determination and put it in the box. O N SH + F3C OH + + _N_ + OH Oo. 8arrow_forwardEach of the following reactions have mistakes in them, please identify those as completely as you can. اخيرة A) B) HI 1.03 Di 2. Zn 1) BH 2) H2O, OH OHarrow_forward
- What is the starting material in the following reaction? IV H₂, Lindlar's cat. Br2 || Br Br IVarrow_forwardIn reaction D: CuO + H2SO4= CUSO4 + H2O Students will occasionally use HNO3 (aq) instead of H2SO4 (aq) in reaction D, assuming that both strong acids will accomplish the same purpose. Briefly describe the results of this error.arrow_forward3. Complete the reactions below: hium aluminium hydride CH,OH Tollen's Reagent Аз PCC (excess) A1 > A2 PHCH,NH, (excessi H Zn(Hg), HCI dilute chromic acid A8 A4 Brz, uv A5 A7 A6 HO. OH OHarrow_forward
- I was super excited to get into the lab and make compound B from Compound A. I refluxed compound A in water and crossed my fingers. Son of a .... it turned out I made Compound C. What kind of reaction was this? Should I have used NaOH in water instead? $=$ H₂O Br OH B НО. 88arrow_forwardWrite the chemical formula for diarsenic trioxide 14- 3+ 4+ 1 4 9. (s) (1) (g) (aq) As An Ar Ox Reset • x H2O Delete 3, 2.arrow_forwardBr 2 Choose the correct reagents from the following list: A В Zn(Hg), HCI, heat (CH3)2CHCI, AICI3 Zn, HCI D E F Br2, AIBR3 HNO3, H2SO4 CH3COCI, AICI3 Enter the correct letters in the boxes given below. (Reagents cannot be used more than once) Reagent 1: Reagent 2: Reagent 3:arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





