
Concept explainers
Compound X
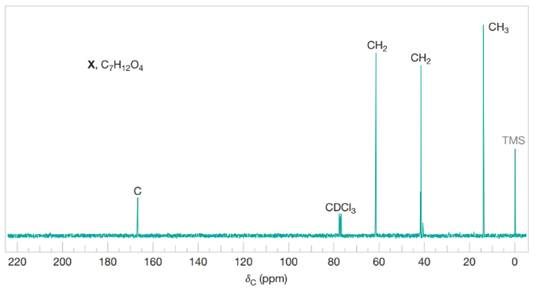
Figure 4 Broadband proton-decoupled
Want to see the full answer?
Check out a sample textbook solution
Chapter SRP Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry For Changing Times (14th Edition)
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
Organic Chemistry As a Second Language: Second Semester Topics
Living by Chemistry
Chemistry (7th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
- 3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forwardAddition of m-xylene to the strongly acidic solvent HF/SbF5 at 45C gives a new species, which shows 1H-NMR resonances at 2.88 (3H), 3.00 (3H), 4.67 (2H), 7.93 (1H), 7.83 (1H), and 8.68 (1H). Assign a structure to the species giving this spectrum.arrow_forwardPropose a structure for D (molecular formula CgH,CIO,) consistent with the given spectroscopic data. 13C NMR signals at 30, 36, 128, 130, 133, 139, and 179 ppm 1Η ΝMR of D 4H 2H 2 H 1H 12 10 8 4 2 ppmarrow_forward
- Given the following 13C NMR signals, construct a structure for the unknown compounds. A. The unknown compound has M+∙ = 86 in its mass spectrum and a broad peak at 3400 cm-1 in its IR spectrum. The following are the 13C NMR spectral data: Broadband decoupled: 30.2 δ, 31.9 δ, 61.8 δ, 114.7 δ, 138.4 δ DEPT-90: 138.4 δ DEPT-135 (positive): 138.4 δ DEPT-135 (negative): 30.2 δ, 31.9 δ, 61.8 δ, 114.7 δarrow_forward15. Compound A, a hydrocarbon with M* = 96 in its mass spectrum, has the ¹³C spectral data given below. On reaction with BH3 followed by treatment with basic H₂O2, A is converted into compound B, whose ¹³C spectral data are also given below. Propose structures for A and B. Compound A Broadband-decoupled ¹³C NMR: 26.8; 28.7; 35.7; 106.9; 149.7 8 DEPT-90: no peaks DEPT 135: no positive peaks; negative peaks at: 26.8; 28.7; 35.7; 106.9 8 Compound B Broadband-decoupled ¹³C NMR: 26.1; 26.9; 29.9; 40.5; 68.2 8 DEPT-90: 40.5 8 DEPT-135: positive peaks at 40.5 8; negative peaks at: 26.1; 26.9; 29.9; 68.2 8arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


