
Concept explainers
Interpretation:The absolute configuration of molecules depicted should be written.
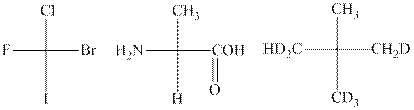
Concept introduction:In order to assign absolute configuration of R and S Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order with the
The Fischer projection is written along with the priorities assigned and groups are interchanged between adjacent places so as to obtain the lowest priority group at the bottom or lowest priority. If the groups now are arranged are read from highest towards least in clockwise fashion then R is assigned to the stereocenter if the rotation is anticlockwise the S is assigned at the configuration.
Any organic compound must have no plane of symmetry in order to be optically active. The compounds with any plane of symmetry are achiral and optically inactive.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: Structure and Function
- Examine the ungraded ball-and-stick model below to determine the three-dimensional structure of the molecule. On the corresponding 2D structure, draw one wedge bond and one dash bond over two existing bonds to indicate the same arrangement of atoms in space. The narrow part of each wedge-and-dash bond should be towards the same central carbon atom.arrow_forward5. Follow the curve arrows in the image below and draw the correct second resonan structure.arrow_forwardUse the dropdown to select the correct description for a molecule of CH₂F2. :F: H-C-H :F: Electron domain geometry: [Select] Molecular geometry: [Select] F-C-H bond angle: [Select] Molecular polarity: [Select] Hybridization on central atom: [Select] Valence Bond Theory description of the sigma bond in C-H bond: [Select] Number of sigma bonds in the molecule: [Select] Number of pi bonds in the molecule: [Select] #arrow_forward
- Draw each molecule with its correct geometry in 3D and indicate the direction of the molecule's overall dipole, if none label it as zero.arrow_forwardFor each compound below, decide whether cis and trans isomers are possible. If isomerism is possible, draw the other isomer.arrow_forwardThe structure of 1,2-propadiene (allene) is shown to the right. (a) Predict all approximate bond angles in this molecule. (b) State the orbital hybridization of each carbon. (c) Explain the three-dimensional geometry of allene in terms of the orbitals used.arrow_forward
- Assign hybridization state of each carbon, nitrogen, oxygen, halogen, and boron atom indicated with arrows in the molecules below.arrow_forwardShow the angles around each atom, except for hydrogens. Draw a complete orbital diagram for the compound shown below, showing all of the sigma and pi bond orbitals in the process.arrow_forwardConsider the molecule below. Determine the molecular geometry at each of the 2 labeled carbons. H H CC=C 1 H CI C=C CH3 F C1 tetrahedral, C2 - linear = O C1 = trigonal planar, C2- bent OC1 trigonal planar, C2 = tetrahedral O C1 = trigonal pyramidal, C2 = see-saw OC1 = bent, C2 = trigonal planararrow_forward
- 10. Which element has the electron configuration 1s² 2s²2p³? C. S d. Cl 11. How many hydrogen atoms are connected to the indicated carbon atom? a. O b. F a. one b. two c. three 12. Which of the following is the correct condensed structure for the following compound? H H H H H H H H-C C -C C -C C C -H HC H H Br H H H H a. CH₂C(CH3)₂CH₂CH₂(CH)BrC(CH3)2 b. CH3CH₂CH3CCH₂CH₂C(CH3)2CHBr c. (CH3)3CCH₂CH₂CH₂BrCHCH3CH3 d. four e. none H 2 d. CH3CH₂CH₂CCH₂CH₂CHBrCHCH₂CH3 e. (CH3)3CCH₂CH₂CHBRCH(CH3)2arrow_forwardGiven Molecular Formula: C6H1203 Compute for the DU and draw the structure of the compound.arrow_forward4. Describe the hybridization of each carbon atom in the following structure.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





