
Concept explainers
(a)
Interpretation: Whether the indicated cyclohexane derivative is chiral or not should be identified.
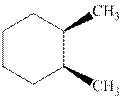
Concept introduction: Four kinds of symmetry elements that may be present are tabulated as follows:
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships. The former are chiral and optically active while the latter can be chiral or achiral.
Any organic compound must have no plane of symmetry in order to be optically active. The compounds with any plane of symmetry are achiral and optically inactive.
(b)
Interpretation: Whether the indicated cyclohexane derivative is chiral or not should be identified.
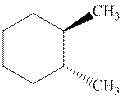
Concept introduction: Four kinds of symmetry elements that may be present are tabulated as follows:
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships. The former are chiral and optically active while the latter can be chiral or achiral.
Any organic compound must have no plane of symmetry in order to be optically active. The compounds with any plane of symmetry are achiral and optically inactive.
(c)
Interpretation: Whether the indicated cyclohexane derivative is chiral or not should be identified.
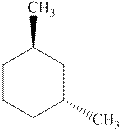
Concept introduction: Four kinds of symmetry elements that may be present are tabulated as follows:
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships. The former are chiral and optically active while the latter can be chiral or achiral.
Any organic compound must have no plane of symmetry in order to be optically active. The compounds with any plane of symmetry are achiral and optically inactive.
(d)
Interpretation: Whether the indicated cyclohexane derivative is chiral or not should be identified.

Concept introduction: Four kinds of symmetry elements that may be present are tabulated as follows:
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships. The former are chiral and optically active while the latter can be chiral or achiral.
Any organic compound must have no plane of symmetry in order to be optically active. The compounds with any plane of symmetry are achiral and optically inactive.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: Structure and Function
- For which isomer would you expect a greater equilibrium percentage of molecules with the alkyl group in the axial position, isopropylcyclohexane or propylcyclohexane? Explain. Isopropylcyclohexane Propylcyclohexanearrow_forwardDraw and name the six isomeric cyclopentane of molecular formula C7H14. These will include four constitutional isomers, of which two show geometric (cis-trans) stereoisomerism.arrow_forwardKetones react with alcohols to yield products called acetals. Why does the all-cis isomer of 4-tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal for each one.arrow_forward
- Consider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardIt is easy to imagine a cyclohexane as a flat hexagon and a lot of the time we draw it that way. Looking at 1,3,5-triethylcyclohexane we cannot tell the stability of the molecule from looking at the flat 2D drawing. Explain why we need to look at the 3D configuration and what conformation (axial,equatorial) would each of the three ethyl groups be in for the most stable configuration.arrow_forwardDraw structural formulas for the cis and trans isomers of hydrindane. Show each ring in its most stable conformation. Which of these isomers is more stable?arrow_forward
- How many unique stereoisomers exist for 1,2,3,4,5-pentachlorocyclohexane?arrow_forwardWhich of the following cyclohexane conformation is (are) consider to be the most stable conformation for cis-1,2-dimethylhexane? I II IIIarrow_forwardUsing what you know about the conformational energetics of substituted cyclohexanes, predict which of the two decalin isomers is more stable. Explain your reasoning.arrow_forward
- The following are representations of two forms of glucose. The six-membered ring is known to exist in a chair conformation in each form. Draw clear representations of the most stable conformation of each. Are they two different conformations of the same molecule, or are they stereoisomers that cannot be interconverted by rotation about single bonds? Which substituents (if any) occupy axial sites?arrow_forwardThe overall process of converting one chair conformation to another chair conformation is known as RING INVERSION or ring flipping and is a very rapid process. The cyclohexane ring inverts approximately 10 time a second at room temperature. Show this interconversion by drawing the two chair conformations and putting a reversible arrow between them to indicate a dynamic equilibrium. If we have a methylcyclohexane molecule and this undergoes ring inversion, whatwill be the resulting structure? ?arrow_forwardDraw all three staggered conformations for the following compound, viewed along the C₂-C3 bond. Determine which conformation is the most stable, taking into account gauche interactions and hydrogen-bonding interactions. (J. Phys. Chem. A 2001, 105, 6991-6997) Provide a reason for your choice by identifying all of the interactions that led to your decision. H₂C OH C₂ HO CH3 Which of the following is the most stable staggered conformer? Me Me *** Conformer B Me- Me Conformer A Me Conformer C Me Conformer B because the anti OH groups avoid hydrogen bonding, which is a destabilizing effect. Conformer A because there are only two gauche interactions, and hydrogen bonding between the two OH groups is a stabilizing effect. Conformer C because there is no steric strain for Me-OH gauche interactions and hydrogen bonding between the two OH groups is a stabilizing effect. Conformer B because the large OH groups are anti to each other.arrow_forward

 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


