
Concept explainers
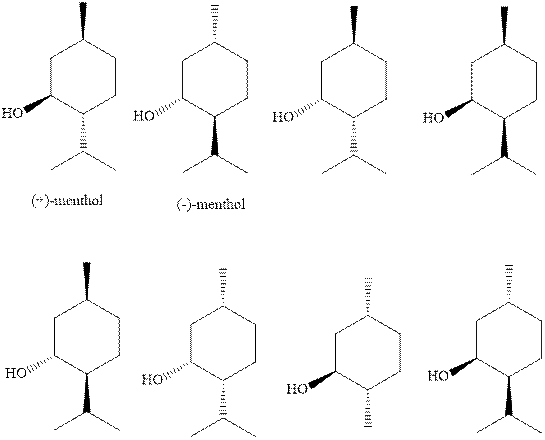
(a)
Interpretation:The natural
Concept introduction:
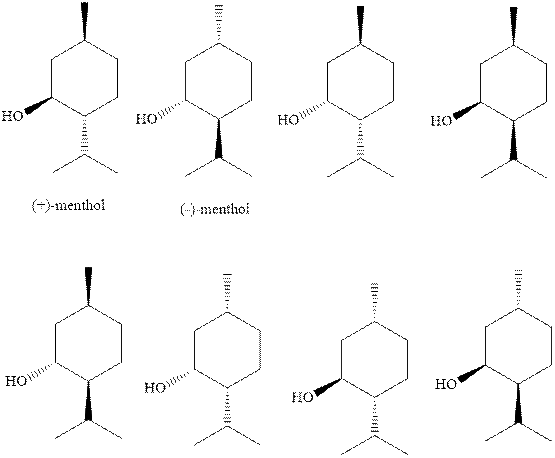
(b)
Interpretation: The natural
Concept introduction:
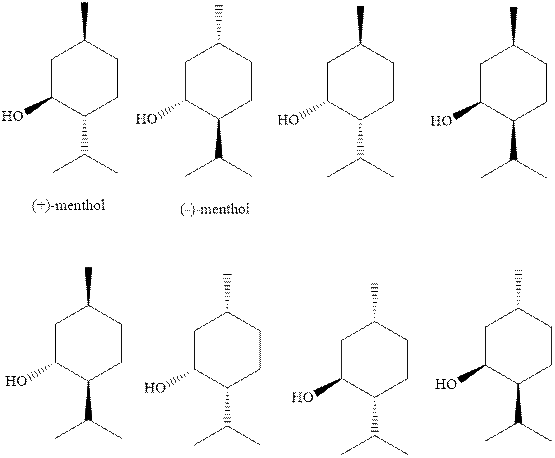
(c)
Interpretation: The natural
Concept introduction:
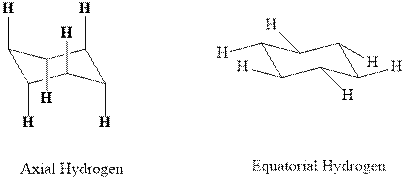
(c)
Interpretation: The stability order for the three diastereomers namely menthol, isomenthol, and neomenthol should be determined.
Concept introduction:In chair form all the
bond further allows for no amount of torsional strain. There are two kinds of hydrogens in cyclohexane namely axial and equatorial indicated as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: Structure and Function
- Department Final EXd Q1: Coibacin B is a natural product that exhibits potent anti-inflammatory activity and potential activity in the treatment of leishmaniasis, a disease caused by certain parasites. HAH H (a) Assign the configuration (R or S) of each chiral center in coibacin B. (b) Identify the number of possible stereoisomers for this compound, assuming that the geometry of the alkenes is fixed.arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Draw a Newman projection for the conformation in which CH3 and -Br are anti (dihedral angle 180°). (b) Draw Newman projections for the conformations in which - CH3 and -Br are gauche (dihedral angles 60° and 300°). (c) Which of these is the lowest energy conformation? (d) Which of these conformations, if any, are related by reflection?arrow_forwardQ1: Draw the structure of each of the following molecules: (a) (R)-1-chloro-1-fluorobutane (c) (2R,35)-3-Bromo-2-chloro-1-pentanol (b) (S)-2-chloropentane; (d) (R)-2,2,3-trichlorobutane.arrow_forward
- Draw a three-dimensional structure for each compound, and star all asymmetric carbon atoms. Draw the mirror image for each structure, and state whether you have drawn a pair of enantiomers or just the same molecule twice. Build molecular models of any of these examples that seem difficult to you. (a) ОН (b) (c) NH, ОН СH—CH—СООН | pentan-2-ol pentan-3-ol alanine (d) 1-bromo-2-methylbutane (e) chlorocyclohexane (f) cis-1,2-dichlorocyclobutane (h) (i) H CH; "H H. H CH, Harrow_forward(E) -1-2- Diphenylethene reacts with Br2 /CCI 4 (see reaction) the reaction product is obtained (A) is a stereoisomer with 2 chiral optically inactive carbons that then reacts with KOH / ethanol (see reaction) that produces an intermediate (B) [C₁4H₁1 Brl that by continuing to react with KOH and heating to 190 degrees Celsius, compound (D) C14H10 is obtained a) Write the general reaction of this synthesis with the structures of the initial alkene of A, B and D b) Give the reaction mechanism to obtain A c) Indicate for the absolute configuration of each C* and the IUPAC name of the compound d) Give the IUPAC and common name of the obtained product Darrow_forwardH Hg(OAc)2, H₂O, THE draw all stereo isomers NaBD4, OH (D = ²H) draw all stereo isomersarrow_forward
- (a) (R)-1,1,2-trimethylcyclohexane, draw any enantiomer.arrow_forwardQ3:- Draw and specify as R or S the enantiomers (if any) of: (a) 3-bromohexane (d) 1,3-dichloropentanearrow_forwardFor each alkene, indicate the direction of the dipole moment. For each pair, determine which compound has the largerdipole moment.(a) cis-1,2-difluoroethene or trans-1,2-difluoroethene(b) cis-1,2-dibromoethene or trans-2,3-dibromobut-2-ene(c) cis-1,2-dibromo-1,2-dichloroethene or cis-1,2-dichloroethenearrow_forward
- Calculate the number of degrees of unsaturation for each molecular formula, and propose two possible structures: (a) C8H12; (b) C10H10.arrow_forwardDraw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?arrow_forwardDraw structures for the following compounds: (a) 2,2-dimethylpropane (b) 3-methylheptane (c) 4,5-diethylnonane (d) 4-(1-methylethyl) heptane (e) 5-(1,1-dimethylethyl) nonane (f) ethylcyclobutane (g) 1-ethyl-4-methylcyclohexanearrow_forward
