
Concept explainers
(a)
Interpretation:The stereocenter in thalidomide should be identified.
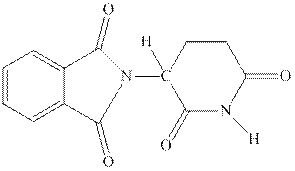
Concept introduction:Chiral carbon is any stereocenter attached to four different alkyl substituents. It is also known as stereocenter. If any two of the substituent happen to be similar the center is regarded as achiral. However, the most essential criteria that help to distinguish a chiral or non-chiral system ispresence of any symmetry element. If any plane center or axis of symmetry is identified it makes molecule achiral and optically inactive.
Thalidomide was originally taken as sedative against morning sickness; while one of its enantiomers was successful as sedative, other enantiomer caused certain birth defects.
(b)
Interpretation:Two enantiomers in thalidomide should be drawn and labeled.
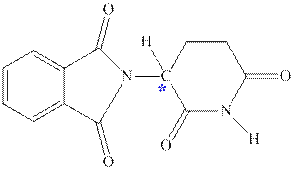
Concept introduction:In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order on the basis of
The Fischer projection is written along with the priorities assigned and groups are interchanged between adjacent places so as to obtain lowest priority group at the bottom or lowest priority.
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
(c)
Interpretation: The relevant hydrogen that shows acidic properties should be identified in thalidomide.
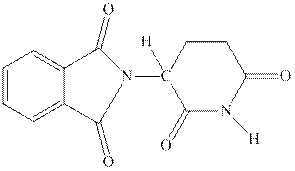
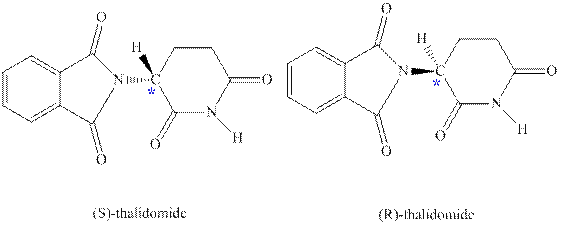
Concept introduction:Thalidomide was originally taken as sedative against morning sickness; while one of its enantiomer was successful as sedative, other enantiomer caused certain birth defects. The two enantiomers of thalidomide are as follows:
Trending nowThis is a popular solution!

Chapter 5 Solutions
Organic Chemistry: Structure and Function
- Azulene, an isomer of naphthalene, has a remarkably large dipole moment for a hydrocarbon (μ = 1.0 D). Explain, using resonance structures.arrow_forwardCompounds A and B are isomers having the molecular formula C4H8O3. Identify A and B on the basis of their 1H NMR spectra.Compound A: δ 1.3 (3H, triplet); 3.6 (2H, quartet); 4.1 (2H, singlet); 11.1 (1H, broad singlet)Compound B: δ 2.6 (2H, triplet); 3.4 (3H, singlet); 3.7 (2H triplet); 11.3 (1H, broad singlet)arrow_forwardCompound A exhibits a peak in its 1H NMR spectrum at 7.6 ppm, indicating that it is aromatic. (a) How are the carbon atoms of the triple bonds hybridized? (b) In what type of orbitals are the π electrons of the triple bonds contained? (c) How many π electrons are delocalized around the ring in A?arrow_forward
- Cyclopropenones are described as having aromatic character. How would you account for this, given that the ring contains three ℼ-electronarrow_forward(A)Menthol, used to flavor various foods and tobacco, is the most stable stereoisomer of 2-isopropyl-5-methylcyclohexanol. Draw its most stable conformation. Is the hydroxyl group cis or trans to the isopropyl group? To the methyl group? (b) Neomenthol is a stereoisomer of menthol. That is, it has the same constitution but differs in the arrangement of its atoms in space. Neomenthol is the second most stable stereoisomer of 2-isopropyl-5methylcyclohexanol; it is less stable than menthol but more stable than any other stereoisomer. Write the structure of neomenthol in its most stable conformation.arrow_forwardDescribe the conformation of rings A, B, C, and D in cholestanol.arrow_forward
- c) An unknown chiral organic compound with molecular formula C7H1402 has a characteristic IR absorptions at approximately 3000 cm1, 1715 cm1, 2400 - 3400 cm-1 (broad absorption) and 1250 cm1. Propose a possible structure of this unknown compound.arrow_forwardd) Identify one compound that is expected to have identical physical properties as structure II. (e) Other than structures III and VI, identify a stereoisomer with a different boiling point from that of structure II. (f) Other than structures IV, identify one diastereomer of structure I. (g) How many stereoisomers may be derived from structure V?arrow_forward(b) Consider Scheme Q21b: (i) OH im i base Scheme Q21b E What type of reaction is taking place that convert D to E? (ii) Redraw structure E then circle the substituents on the double assign priorities according to the Cahn-Ingold-Prelog rules. (iii) Derive the IUPAC name of structure E.arrow_forward
- 5.69 Carvone is present in many essential oils. It is chiral, and its two enantiomers are shown here. Even though they are enantiomers, they have different odors. The (-) enantiomer smells like spearmint, whereas the (+) enantiomer smells like caraway seeds. What does this say about the olfactory receptors that detect carvone? than a plana) HROO HO HO HO H00 osses O= Datum his H. HO HO HO Tefur OH Hie ane 2000 (-)-Carvone OH muistad Y (+)-Carvone HO yet it pelesses no Harrow_forwardRank by the stability of the alkene isomers. The most stable isomer is 1, while the least stable isomer is 5. (A) (B) (C) (D) (E)arrow_forwardConsider the reaction between (1S,3S)‑1‑chloro‑3‑methylcyclopentane and methanethiol in the presence of sodium hydroxide. (a) Draw the organic product and clearly indicate stereochemistry by showing the hydrogen on the chirality centers and using wedge and dash bonds. (b) Then analyze the stereochemistry of the product. racemic chiral achiral (1R, 3S) (1R, 3R) (1S, 3S)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

