
Concept explainers
Interpretation: Either of one of the enantiomeric forms of below chiral molecules should be drawn and stereochemistry of carbon should be labeled.
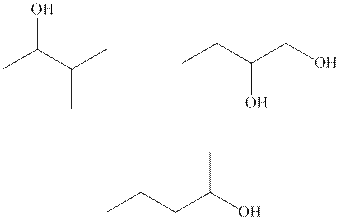
Concept introduction: In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order on the basis of
The Fischer projection is written along with the priorities assigned and groups are interchanged between adjacent places so as to obtain lowest priority group at the bottom or lowest priority.
If the groups arranged are read from highest towards least in clockwise fashion then R is assigned to the stereocenter.If the rotation is anticlockwise then S is assigned at the configuration.
The enantiomers are identical chemical compounds that have a mirror-image relationship to each other while diastereomers do not hold mirror image relationships. The former are chiral and optically active while the latter can be chiral or achiral.
Any organic compound must have no plane of symmetry in order to be optically active. The compounds with any plane of symmetry are achiral and optically inactive.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: Structure and Function
- Draw all possible stereoisomers for HC=OCHClCHClCH2Br. Label the compounds as Compound I, II, etc.. Determine the relation of each of these compounds to another (pair relations as enantiomers, diastereomer, mesocompound or no relations).arrow_forwardAssign (R) or (S) configuration to each chiral center in the molecules below. Then indicate if the molecules are identical, constitutional isomers, enantiomers, or diastereomers. F F ОН VS. OHarrow_forwardThe structure of 4 isomers of ketopentose are shown. 1) select every structure that is a diastereomer of structure D A, B, or C? 2) select every structure that is a enantiomer of structure C A, B, or D 3) select every structure that is a stereoisomer of structure C A, B, or Darrow_forward
- 5. Consider the following pairs of structures. Identify the relationship between them (enantiomers, diastereomers, or identical compounds. он HO o CH,OH H,C H но CH,OH сон H HO. HO CH, HO,C CH COH HO,C, CO,H CO,H н онн он н онн он HOH,C сно OHC CH,OH Br CH,CH, H,CH,C CI CH OHarrow_forward5. Consider the following pairs of structures. Identify the relationship between them (enantiomers, diastereomers, or identical compounds. HOH2C H OH H OH HO H Br H3C Н H H₂CH₂C CI Br H CHO CH3 I CH3 OH OHC Н H OH H OH Bell!! H3C, HO H Н.С H CH₂CH3 CH3 CI HỌ,H CH₂OH Brarrow_forwardHow many chiral centers are there in this molecule and identify them on the image? Is it delivered as one enantiomer or a mixture?arrow_forward
- NH2 N CH2 CH2 The stereochemistry around this chiral carbon is: O cannot be determined. O Rand S OR OSarrow_forwardIdentify the configuration of each chiral center in the following compounds. Draw the enantiomer for each of the following compoundsarrow_forward2. Draw the optical isomers of CH3-CHOH-CHOH-CH3 . INDICATE the absolute configuration of each chiral center. Which are enantiomers? Which are diastereomers? Is there any meso compound present? Identify if yes.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

