
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.11P
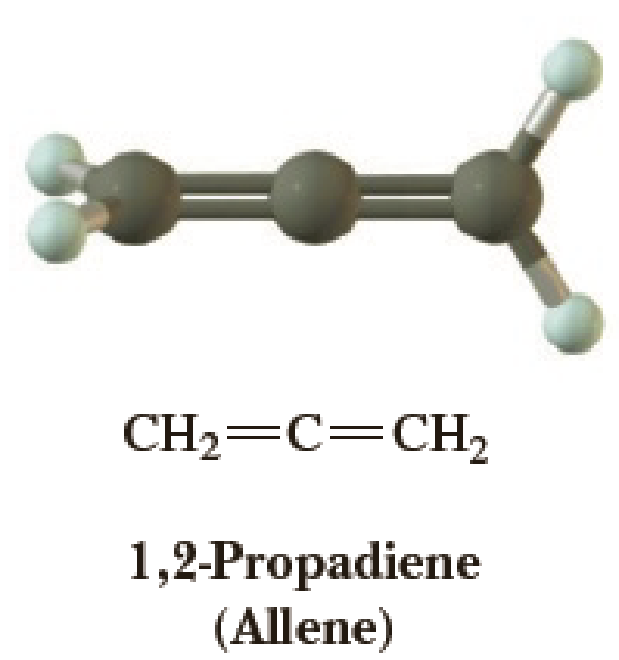
The structure of 1,2-propadiene (allene) is shown to the right.
- (a) Predict all approximate bond angles in this molecule.
- (b) State the orbital hybridization of each carbon.
- (c) Explain the three-dimensional geometry of allene in terms of the orbitals used.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
H2CO molecules
(a) use orbital hybridization theory to determine the molecular shape of h2co.
(b) what bonds are formed between the c and o atoms in formaldehyde molecules?
The structure of caffeine is shown below.
(a) Complete the Lewis structure.
(b) How many pi bonds are present in caffeine? How many sigma bonds?
(c) Identify the hybridization of the carbon atoms.
(d) What is the value of the O-C-N angle?
Describe the molecular geometry and hybridization of the N, P, or S atoms in each of the following compounds.(a) H3PO4, phosphoric acid, used in cola soft drinks(b) NH4NO3, ammonium nitrate, a fertilizer and explosive(c) S2Cl2, disulfur dichloride, used in vulcanizing rubber(d) K4[O3POPO3], potassium pyrophosphate, an ingredient in some toothpastes
Chapter 5 Solutions
Organic Chemistry
Ch. 5.1 - Calculate the index of hydrogen deficiency for...Ch. 5.1 - Prob. 5.2PCh. 5.2 - Write the IUPAC name of each alkene. (a) (b)Ch. 5.2 - Prob. 5.4PCh. 5.2 - Prob. 5.5PCh. 5.2 - Prob. 5.6PCh. 5.2 - Prob. 5.7PCh. 5.2 - Prob. 5.8PCh. 5 - Predict all approximate bond angles about each...Ch. 5 - Prob. 5.10P
Ch. 5 - The structure of 1,2-propadiene (allene) is shown...Ch. 5 - Prob. 5.12PCh. 5 - Draw structural formulas for these alkenes. (a)...Ch. 5 - Name these alkenes and cycloalkenes.Ch. 5 - Prob. 5.15PCh. 5 - Prob. 5.16PCh. 5 - Prob. 5.17PCh. 5 - For each molecule that shows cis, trans isomerism,...Ch. 5 - -Ocimene, a triene found in the fragrance of...Ch. 5 - Prob. 5.20PCh. 5 - Prob. 5.21PCh. 5 - Prob. 5.22PCh. 5 - Prob. 5.23PCh. 5 - Prob. 5.24PCh. 5 - Measure the CH3,CH3 distance in the...Ch. 5 - Prob. 5.26PCh. 5 - Measure the CCC and CCH bond angles in the...Ch. 5 - Prob. 5.28PCh. 5 - Prob. 5.29PCh. 5 - Prob. 5.30PCh. 5 - Prob. 5.31PCh. 5 - Prob. 5.32PCh. 5 - Prob. 5.33PCh. 5 - Pyrethrin II and pyrethrosin are two natural...Ch. 5 - Prob. 5.35PCh. 5 - Prob. 5.36PCh. 5 - Bromine adds to cis- and trans-2-butene to give...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe the molecular geometry and hybridization of the N, P, or S atoms in each of the following compounds. (a) H3PO4, phosphoric acid, used in cola soft drinks (b) NH4NO3, ammonium nitrate, a fertilizer and explosive (c) S2Cl2, disulfur dichloride, used in vulcanizing rubber (d) K4[O3POPO3], potassium pyrophosphate, an ingredient in some toothpastesarrow_forwardIdentify any carbon atoms that change hybridization and the change in hybridization during the reactions in (a) 2-butene reacts with chlorine.(b) benzene burns in air.arrow_forwardFor each statement, indicate whether it is true or false. (a) The greater the orbital overlap in a bond, the weaker the bond. [b] The greater the orbital overlap in a bond, the shorter the bond. [c] To create a hybrid orbital, you could use the s orbital on one atom with a p orbital on another atom. [d] Nonbonding electron pairs cannot occupy a hybrid orbital.arrow_forward
- (a) Describe the hybridization of the central atom of a molecule with a see-saw shape. (b) Describe the hybridization of the central atom of a molecule with a trigonal planar shape. (c) Describe the hybridization of the central atom of a molecule with a trigonal bipyramidal shape.arrow_forwardDraw a Lewis electron dot diagram for each of the follow- ing molecules and ions. Formulate the hybridization for the central atom in each case and give the molecular geometry. (a) BF3 (b) BH4- (c) PH3 (d) CS2 (e) CH3+arrow_forwardOrganic compounds (a) A simple molecule with alternating single and double bonds is buta-1,3-diene. (i) Draw its skeletal structure. (ii) Write its molecular formula and empirical formula. (iii) How many carbon atoms have sp2 hybridization in buta-1,3-diene?arrow_forward
- (a) Find the angle u between adjacent nearest-neighbor bonds in the silicon lattice. Recall that each silicon atom is bonded to four of its nearest neighbors.The four neighbors form a regular tetrahedron— a pyramid whose sides and base are equilateral triangles. (b) Find the bond length, given that the atoms at the corners of the tetrahedron are 388 pm apart.arrow_forward(a) Sketch the molecular orbitals of the H2- ion and draw itsenergy-level diagram. (b) Write the electron configuration ofthe ion in terms of its MOs. (c) Calculate the bond order inH2-. (d) Suppose that the ion is excited by light, so that anelectron moves from a lower-energy to a higher-energy molecularorbital. Would you expect the excited-state H2- ion to bestable? (e) Which of the following statements about part (d) is correct: (i) The light excites an electron from a bonding orbitalto an antibonding orbital, (ii) The light excites an electronfrom an antibonding orbital to a bonding orbital, or (iii)In the excited state there are more bonding electrons thanantibonding electrons?arrow_forward(a) State the hybrid orbitals for C1,C2,O1 and O2 (b) state the number of π bonds present (c) State teh number of C atoms with sp2 hybridization.arrow_forward
- Butadiene, C4H6, is a planar molecule that has the followingcarbon–carbon bond lengths: (a) Predict the bond angles around each of the carbon atoms and sketch the molecule. (b) From left to right, what is the hybridization of each carbon atom in butadiene? (c) The middle C—C bond length in butadiene (1.48 Å) is a little shorter than the average C—C single bond length (1.54 Å). Does this imply that the middle C—C bond in butadiene is weaker or stronger than the average C—C single bond? (d) Based on your answer for part (c), discuss what additional aspects of bonding in butadiene might support the shorter middle C—C bond.arrow_forwardExplain why(a) XeF2 has a linear molecular structure and not a bent one.(b) ClF3 has a T-shaped structure and not a trigonal-planar one.arrow_forwardIn the hydrocarbon: (a) What is the hybridization at each carbon atom in themolecule? (b) How many σ bonds are there in the molecule?(c) How many π bonds? (d) Identify all the 120° bond anglesin the molecule.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY