
Concept explainers
(a)
Interpretation:
The structure of the salmeterol should be drawn.
Concept Introduction:
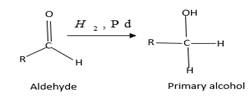
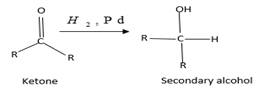
(b)
Interpretation:
One chirality center in a salmeterol molecule should be identified.
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image. To consider as chiral, molecule or object and its mirror image should not superimpose. To consider as achiral, molecule or object and its mirror image should be superimposed with each other.
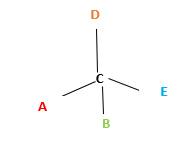
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different
(c)
Interpretation:
Enantiomers of salmeterol in three dimensions should be drawn.
Concept Introduction:
Mirror image of a molecule is a reflected duplicate of the molecule.
Enantiomers − the mirror image of the original molecule of a chiral molecule. These are stereoisomers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
General, Organic, and Biological Chemistry - 4th edition
- 1. Draw a pair of enantiomers of 2-butanol. Is 2-butanol able to rotate the plane polarized light? Explain.arrow_forwardDraw the structure for a compound with molecular formula C2H2I2F2 a. that is optically inactive because it does not have an asymmetric center. b. that is optically inactive because it is a meso compound. c. that is optically active.arrow_forwardEphedrine, a stimulant and illegal drug is an optically active compound with two chiral centers. OH NHCH3 CH3 Ephedrine a. In the structure of ephedrine on the answer sheet, mark each chiral center with an asterisk. b. How many stereoisomer/s does ephedrine have? Show necessary solutions on the answer sheet. c. Compound EPS is a stereoisomer of ephedrine. Determine the absolute configuration (R or S) at carbons A and B of compound EPS.arrow_forward
- III. Consider the compound below. H. F HO- HO -HO- (Atomic number: H-1, C-6, O-8, F-9) a. How many chiral centers are in the molecule? b. How many stereoisomers are there for the compound? c. Draw the Fischer projection for each of the stereoisomer and determine the absolute configuration (R or S). Label each using I, II, etc. d. Which pairs are enantiomers? Which are diastereomers?arrow_forward#544 of Isomers (chiral) Which of the following 2-chloro-1-butanol (A), 3-chloro-1-butanol (B), and 4-chloro-1-butanol (C), contain a stereogenic carbon atom. Select one: a. A only O b. B only O c. C only O d. A and B e. A, B and Carrow_forward4. ) Determine if the following compound is chiral or not. OH HO OH Me ZOH Ме HO HO OH F. CI Ме CI Он Me JCHO CI Br Br CI CH3arrow_forward
- Because there is usually slow interconversion between the two isomeric forms at room temperature. Because there is usually rapid interconversion between the two isomeric forms at room temperature. Because chirality only exists with the tetrahedral carbon atoms. Because four bonds a are needed to define a stereogenic center.arrow_forwardDrawn are four isomeric dimethylcyclopropane. a. How are the compounds in each pair related (enantiomers, diastereomers,constitutional isomers): A and B; A and C; B and C; C and D?b. Label each compound as chiral or achiral.c. Which compounds, alone, would be optically active?d. Which compounds have a plane of symmetry?e. Which of the compounds are meso compounds?f. Would an equal mixture of compounds C and D be optically active? Whatabout an equal mixture of B and C?g. How many stereogenic centers are there for each compound?arrow_forwardball & stick + labels stereoisomer(s) e. Are the two molecules enantiomers? yes no ball & stick a. How many chiral carbons are there in the molecule on the left? chiral carbon(s) b. What is the total number of stereoisomers in the group that includes the molecule on the left? stereoisomer(s) c. How many chiral carbons are there in the molecule on the right? chiral carbon(s) d. What is the total number of stereoisomers in the group that includes the molecule on the right? + labels Previous Next Save and Exitarrow_forward
- 2. What is the relationship between these two molecules? o a. Identical b. Constitutional isomers c. Enantiomers d. Diastereomersarrow_forwardThis is one enantiomer of the molecule. OH H CCH2-CH2-CH3 CH3 Draw the structure of the other enantiomer using wedges and dashes. Click and drag to start drawing a structure.arrow_forwardSaquinavir (trade name Invirase) belongs to a class of drugs called protease inhibitors, which are used to treat HIV (human immunodeficiency virus). OH CONH2 O saquinavir Trade name: Invirase NH a. Locate all stereogenic centers in saquinavir, and label each stereogenic center as R or S. b. Draw the enantiomer of saquinavir. c. Draw a diastereomer of saquinavir. d. Draw a constitutional isomer that contains at least one different functional group.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





