
Concept explainers
a.
Interpretation:
The IUPAC name of given compounds has to be drawn.
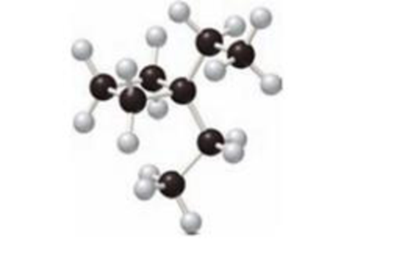
Concept Introduction:
The system which is used to name an organic compound is known as IUPAC Nomenclature. IUPAC are known as International Union of Pure and Applied Chemistry.
There are some rules followed for writing IUPAC Nomenclature for
- The longest chain in the compound is known as parent name which represent the number of carbon atom present in the continuous carbon chain. The root name for alkane compound is –ane. The single bonds are formed in alkane compounds.
- The suffix group denotes the
functional group present in a molecule. The prefix group indicates the identity, location and number of substituents attached to the carbon compound. - Then name and number the substituents present in compounds. Then use prefix di-to represent two groups, tri- refers to three groups and so on. The numbers can be separated by using commas and the letters from numbers can be separated by using dashes.
b.
Interpretation:
The one constitutional isomer of given compound has to be drawn.
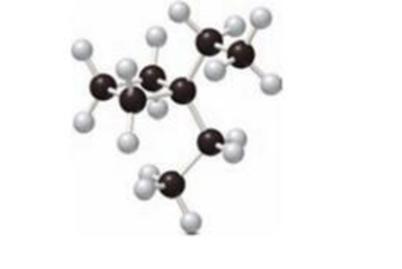
Concept Introduction:
Isomers:
The organic compound contains same molecular formula and different arrangement of atoms is termed as isomers. The isomers have different physical and chemical properties.
Constitutional Isomer:
Constitutional isomer has same molecular formula but they have different connectivity to each other. It is also known as structural isomers.
c.
Interpretation:
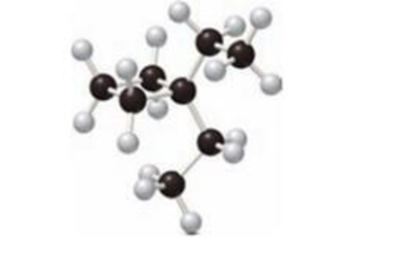
The solubilty of given compounds in water has to be predicted.
d.
Interpretation:
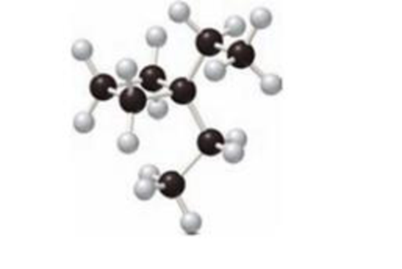
The solubilty of given compounds in organic solvent has to be predicted.
e
Interpretation:
The skeletal structure of the given compound has to be drawn.
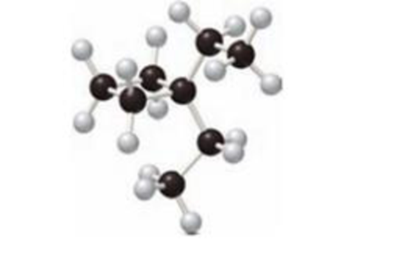
Concept Introduction:
Skeletal Structures:
The skeletal structure is the diagrammatic representation of organic compounds. It is used for compounds containing rings and chain of compounds. The bonds of atoms bonded together to form a skeletal structure. There is no need to mention the carbon and hydrogen element. There is no need to mention the lone pairs of electrons present in heteroatoms. The heteroatom has to be drawn and the hydrogen is bonded directly to them.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Principles of General, Organic, Biological Chemistry
- 9. Select the correct IUPAC name for : H,C- .CH, A. 1,4-dimethylcyclopentane. B. 1,3-dimethylcyclopentane. C. 2,5-dimethylcyclopentane. D. 2,3-dimethylcyclopentane. Agoula E. 2,4-dimethylcyclopentane. nobarrow_forward1. Draw the structure of each of the following cycloalkanes a. 1-Bromo-2-methylcyclobutaneb. 1,2-Dibromo-3-methylcyclohexanec. Iodocyclopropane2. How does the general formula of a cycloalkane compare to that of an alkane?arrow_forward1. a. Draw the structures of the two isomers of dibromoethane. Name them by the IUPAC system. b. Draw out the structures of the two isomers of iodopropane. Name them by the IUPAC system. c. Draw the structures of the three isomers of dichloroethene. Name each one by the IUPAC system, indicating stereochemistry where relevant.arrow_forward
- Choose the correct statement about the molecules above. a. It is not soluble in polar solvents b. They have a carboxyl group attached to alkyl grup and H. c. Have a lower boiling point than alcohol d. Have a lower boiling point than alkanes.arrow_forward1. What is the IUPAC name of the compound below? (Please refer to the image attached.) A. 2,2,5-pentylcyclohexane B. 1-Cyclohexyl-2,2,5-trimethylhexane C. 1-Cyclohexyl-1,1,4-trimethylpentane D. 2-Cyclohexyl-2,5-dimethylhexane E. None of the choices 2. The –ene ending suffix of alkene indicates that there is a presence of carbon-carbon _______ bonds in the organic compound. A. Single B. Double C. Triple D. Quadruple 3. It is an acyclic unsaturated hydrocarbon that contains one or more carbon–carbon triple bonds: A. Alkene B. Cycloalkene C. Alkyne D. Cycloalkynearrow_forward1) Write the structure of the indicated isomers. Give their IUPAC names. a. All possible chain isomers of (CH,), C b. Position isomers of cyclohexenol c. Functional isomer of acetophenone d. Functional isomer of butanethiol 2. Give the structure and IUPAC names of all possible skeletal isomers of 1-heptyne.arrow_forward
- Write molecular formulas for each bicycloalkane, given its number of carbon atoms. Q.) Decalin (10 carbons)arrow_forward6. Identify the alkane with the third highest boiling point and the alkane with the second highest boiling point out of the alkanes given below. +arrow_forward2. What is the IUPAC name of the following compound? CH3 a. 2,4-dimethylhexane b. 2-ethyl-4-methylpentane c. 2,4-methylhexane d. 3,5-dimethylhexane e. 2,4-octane H3C-CH-CH2-CH–CH2-CH3 ČH3arrow_forward
- 3. What is the IUPAC name for compound below? 6-Ethyl-3,4-dimethylheptane a. b. 2-Ethyl-4,5-dimethylheptaneoctane c. 3,5,6-Trimethyloctane d. 3,4,6-Trimethyloctane 4. Complete the missing reagent: a. Zn/Hg/HCI b. NH2NH2/ NaOH c. Ni/ HC1 d. Either Zn/Hg/ HCl or NH2NH2/NaOH VENEZINHZ / NaOH 5. Complete the missing reagent: Br H₂C CH CH3 a. Zn/Hg/HCI b. NH2NH2 / NaOH c. Ni/HCI d. Zn/CH3COOH (a) H₂C 6. The IUPAC name of the following; a. 2-Ethyl-1,1-dimethylcyclohexane b. 1-Ethyl-2,2-dimethylcyclohexane c. 1,1-Dimethyl-2-ethylcyclohexane d. 2,2-Dimethyl-1-ethyleveloberans H₂ (b) 10. Which structure does not represent 2-methylpentane? (c) CH3 (d)arrow_forward1. The following are isomers of each other I. pentane II. 2,2,-dimethylpropane III. 2-methylbutaneIV. 2,3-dimethylhexane A. Alkanes are nonpolar. B. Alkanes are non-flammable. C. Every carbon in an alkane has four bonds. D. Alkanes contain only C and H atoms. E. Alkanes do not contain carbon-carbon double or triple bonds. 1b. Which of these is a weak base? A. NaOH B. H3PO4 C. CH3COOH D. NaHCO3 E. NH4NO3 1c. Which of these pairs are formed when NaHCO3 (baking soda) is dissolved in water? NaHCO3 → ______ + ______. A. NaOH + CO2 B. Na+ +HCO3- C. NaH+ +CO3- D. Na + HCO3 E. NaH- + CO3+ 1D. Which of these statements about water is(are) incorrect? A. It has a pH of 7.0 at ambient temperatures. B. It contains no ions. C. It is neutral. D. It can act as a proton donor. E. It can act as a proton acceptor.arrow_forwardChemistry 3. Discuss the physical properties of aliphatic hydrocarbons based on: a. Boiling and melting point of alkanes (as affected by increasing number of carbon atoms) b. Alkane solubility (as affected by increasing number of carbon atoms) c. Combustion Reactions of Alkanes (Example of Reaction and Use)arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
