
Principles of General, Organic, Biological Chemistry
2nd Edition
ISBN: 9780073511191
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 10.53AP
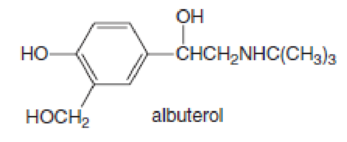
Albuterol (trade names: Proventil and Ventolin) is a bronchodilator, a drug that widens airways, thus making it an effective treatment for individuals suffering from asthma. Draw a complete structure for albuterol with all atoms and lone pairs.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a structure using wedges and dashes for the following compound:
H-
Et
OH
HO-
H
H-
Me
OH
Which of the following molecules are NOT typical carbohydrates? For the molecules that are
carbohydrates, label them as an aldose or ketose.
HO
Он
ОН ОН
Он
ОН
но
ΤΗ
HO
ОН
HO
eve
Он он
ОН
ОН
ОН
If polyethylene has an average molecular weight of 25,000 g/mol, how many repeat units
are present?
Draw the a-anomer cyclized pyranose Haworth projection of the below hexose. Circle the
anomeric carbons. Number the carbons on the Fischer and Haworth projections. Assign R
and S for each chiral center.
HO
CHO
-H
HO
-H
H-
-OH
H
-OH
CH₂OH
Draw the ẞ-anomer cyclized furanose Haworth projection for the below hexose. Circle the
anomeric carbons. Number the carbons on the Fischer and Haworth projections.
HO
CHO
-H
H
-OH
HO
-H
H
-OH
CH₂OH
Chapter 10 Solutions
Principles of General, Organic, Biological Chemistry
Ch. 10.1 - Prob. 10.1PCh. 10.2 - Fill in all Hs and lone pairs in each compound. a....Ch. 10.3 - Convert each compound to a condensed formula.Ch. 10.3 - Convert each condensed formula to a complete...Ch. 10.3 - Convert each skeletal structure to a complete...Ch. 10.3 - How many Hs are bonded to each indicated carbon...Ch. 10.4 - Identify the functional groups in each compound....Ch. 10.4 - For each compound: [1] Identify the functional...Ch. 10.4 - Prob. 10.9PCh. 10.4 - Prob. 10.10P
Ch. 10.4 - Convert the ball-and-stick model of the local...Ch. 10.5 - How many hydrogen atoms are present in each...Ch. 10.5 - Prob. 10.13PCh. 10.5 - Prob. 10.14PCh. 10.5 - Prob. 10.15PCh. 10.5 - Prob. 10.16PCh. 10.6 - Give the IUPAC name for each compound.Ch. 10.6 - Prob. 10.18PCh. 10.6 - Prob. 10.19PCh. 10.6 - Prob. 10.20PCh. 10.7 - Prob. 10.21PCh. 10.7 - Prob. 10.22PCh. 10.9 - Answer the following questions about pentane...Ch. 10.9 - Prob. 10.24PCh. 10.9 - Prob. 10.25PCh. 10.10 - Prob. 10.26PCh. 10 - Prob. 10.27UKCCh. 10 - Prob. 10.28UKCCh. 10 - Prob. 10.29UKCCh. 10 - Prob. 10.30UKCCh. 10 - Prob. 10.31UKCCh. 10 - The largest known cycloalkane with a single ring...Ch. 10 - Draw three constitutional isomers having molecular...Ch. 10 - Draw four constitutional isomers having molecular...Ch. 10 - Answer the following questions about the alkane...Ch. 10 - Answer the questions in Problem 10.35 for the...Ch. 10 - Prob. 10.37UKCCh. 10 - Procaine (trade name Novocain) is a local...Ch. 10 - Prob. 10.39APCh. 10 - Prob. 10.40APCh. 10 - Complete each structure by filling in all Hs and...Ch. 10 - Prob. 10.42APCh. 10 - Prob. 10.43APCh. 10 - Prob. 10.44APCh. 10 - Prob. 10.45APCh. 10 - Prob. 10.46APCh. 10 - Convert each compound to a condensed structure.Ch. 10 - Prob. 10.48APCh. 10 - Prob. 10.49APCh. 10 - Prob. 10.50APCh. 10 - Prob. 10.51APCh. 10 - Prob. 10.52APCh. 10 - Albuterol (trade names: Proventil and Ventolin) is...Ch. 10 - Prob. 10.54APCh. 10 - Prob. 10.55APCh. 10 - Prob. 10.56APCh. 10 - Prob. 10.57APCh. 10 - Prob. 10.58APCh. 10 - Prob. 10.59APCh. 10 - Prob. 10.60APCh. 10 - Prob. 10.61APCh. 10 - Prob. 10.62APCh. 10 - Prob. 10.63APCh. 10 - Prob. 10.64APCh. 10 - Prob. 10.65APCh. 10 - Prob. 10.66APCh. 10 - Prob. 10.67APCh. 10 - Prob. 10.68APCh. 10 - Prob. 10.69APCh. 10 - Prob. 10.70APCh. 10 - Give the IUPAC name for each compound.Ch. 10 - Prob. 10.72APCh. 10 - Prob. 10.73APCh. 10 - Prob. 10.74APCh. 10 - Prob. 10.75APCh. 10 - Prob. 10.76APCh. 10 - Give the structure corresponding to each IUPAC...Ch. 10 - Prob. 10.78APCh. 10 - Prob. 10.79APCh. 10 - Each of the following IUPAC names is incorrect....Ch. 10 - Prob. 10.81APCh. 10 - Prob. 10.82APCh. 10 - Prob. 10.83APCh. 10 - Prob. 10.84APCh. 10 - Prob. 10.85APCh. 10 - Prob. 10.86APCh. 10 - Write a balanced equation for the incomplete...Ch. 10 - Prob. 10.88APCh. 10 - Prob. 10.89APCh. 10 - Prob. 10.90APCh. 10 - Prob. 10.91APCh. 10 - Prob. 10.92APCh. 10 - Answer the following questions for the cycloalkane...Ch. 10 - Prob. 10.94APCh. 10 - Prob. 10.95CPCh. 10 - Prob. 10.96CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the below disaccharide. Circle any hemiacetals. Identify the numbering of glycosidic linkage, and identify it as a or ẞ. OH HO HO OH HO HO HO OHarrow_forwardWhat are the monomers used to make the following polymers? F. а. b. с. d. Вецер хочому なarrow_forward1. Propose a reasonable mechanism for the following transformation. I'm looking for curved mechanistic arrows and appropriate formal charges on intermediates. OMe MeO OMe Me2N NMe2 OTBS OH xylenes OMe 'OTBSarrow_forward
- What is the polymer made from the following monomers? What type of polymerization is used for each? а. ОН H2N но b. ن -NH2 d. H₂N NH2 довarrow_forwardCondensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forwardWhat is the structure of the monomer?arrow_forward
- → BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forwardWhich representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forward
- Please answer 1, 2 and 3 on the endarrow_forwardIn the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forwardOn the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY