
Concept explainers
(a)
Interpretation: The type of isomerism that could be exhibited by the given formulas.
An example that illustrates the specific type of isomerism is to be stated.
Concept introduction: Organic compounds which have similar chemical formula but different structures, i.e., the atoms are in a different spatial arrangement, they are known as structural isomers. Cis and Trans isomers are also structural or geometrical isomers.
To determine: The type of isomerism that could be exhibited by
(a)
Explanation of Solution
Explanation
The structural isomer of
The given formula,
The root word hexene indicates that six carbon atoms are present in the parent chain. Double bond is present between first and second carbon.
The structure of

Figure 1
The structural isomer of
The given formula,
The root word hexene indicates that six carbon atoms are present in the parent chain. The number
The structure of

Figure 2
The structural isomer of
The given formula,
The root word hexene indicates that six carbon atoms are present in the parent chain. The number
The structure of

Figure 3
The structural isomer of
The given formula,
The root word pentene indicates that five carbon atoms are present in the parent chain. The number
The structure of
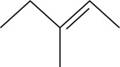
Figure 4
The structural isomer of
The given formula,
The root word butene indicates that six carbon atoms are present in the parent chain. The double bond is present between second and third carbon. Two methyl groups are attached at second and third carbon.
The structure of
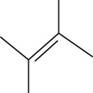
Figure 5
The geometric isomer of
The structure of cis-
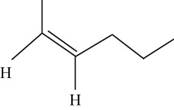
Figure 6
The structure of trans-
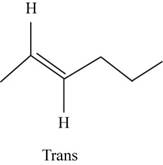
Figure 7
In the cis isomer, the two hydrogen of the double bonded carbons are adjacent to each other while in trans isomer, they are opposite to each other.
The geometric isomer of
The structure of cis-
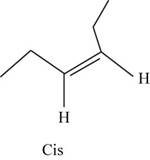
Figure 8
The structure of trans-
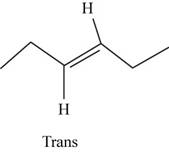
Figure 9
In the cis isomer, the two hydrogen of the double bonded carbons are adjacent to each other while in trans isomer, they are opposite to each other.
(b)
Interpretation: The type of isomerism that could be exhibited by the given formulas.
An example that illustrates the specific type of isomerism is to be stated.
Concept introduction: Organic compounds which have similar chemical formula but different structures, i.e., the atoms are in a different spatial arrangement, they are known as structural isomers. Cis and Trans isomers are also structural or geometrical isomers.
To determine: The type of isomerism that could be exhibited by
(b)
Explanation of Solution
Explanation
The root word pentane indicates that five carbon atoms are present in the parent chain. Hydroxyl group is attached to first carbon.
The structure of pentan-1-ol is shown in Figure 10.
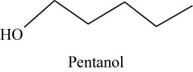
Figure 10
The structural isomer of
The root word butane indicates that four carbon atoms are present in the parent chain.
The structure of
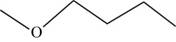
Figure 11
The structural isomer of
The root word propane indicates that three carbon atoms are present in the parent chain.
The structure of
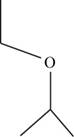
Figure 12
The structural isomer of
The root word butane indicates that four carbon atoms are present in the parent chain.
The structure of
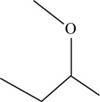
Figure 13
The structural isomer of
The root word pentane indicates that five carbon atoms are present in the parent chain. Hydroxyl group is attached to third carbon.
The structure of pentan-
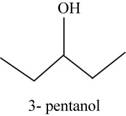
Figure 14
The structural isomer of
The root word pentane indicates that five carbon atoms are present in the parent chain. Hydroxyl group is attached to second carbon.
The structure of pentan-
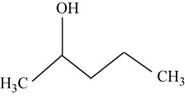
Figure 15
(c)
Interpretation: The type of isomerism that could be exhibited by the given formulas.
An example that illustrates the specific type of isomerism is to be stated.
Concept introduction: Organic compounds which have similar chemical formula but different structures, i.e., the atoms are in a different spatial arrangement, they are known as structural isomers. Cis and Trans isomers are also structural or geometrical isomers.
To determine: The type of isomerism that could be exhibited by
(c)
Explanation of Solution
Explanation
The structural isomer of
In
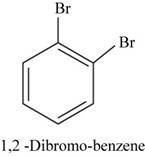
Figure 16
The structural isomer of
In
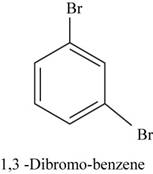
Figure 17
The structural isomer of
In
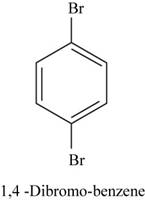
Figure 18
Want to see more full solutions like this?
Chapter 21 Solutions
Chemistry: An Atoms First Approach
- The Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Only 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardPart A Give the IUPAC name and a common name for the following ether: CH3-CH2-O-CH2-CH2-CH3 Spell out the full names of the compound in the indicated order separated by a comma. Submit My Answers Give Up Part B Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma. Submit My Answers Give Uparrow_forwardFrenkel and Schottky are intrinsic or extrinsic defects, point or linear defects.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning


