
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 17.9, Problem 17.6P
Interpretation Introduction
Interpretation: It should be accounted for the observation that the given
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
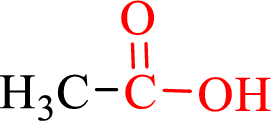
If
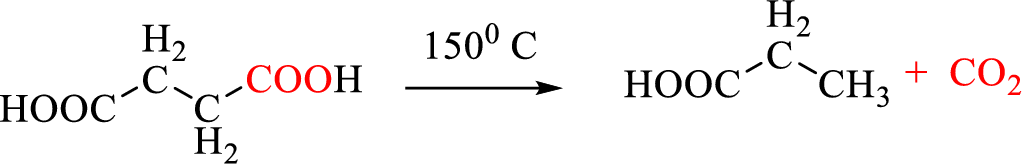
Decarboxylation of
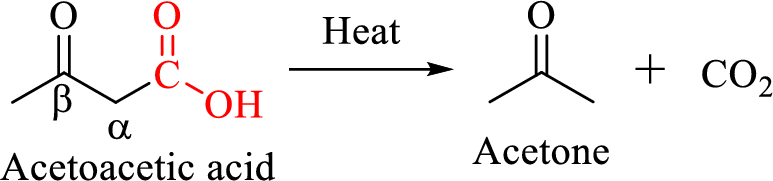
The mechanism of thermal decarboxylation involves two processes,
- (i) Redistribution of electrons in a cyclic transition state.
- (ii) Cyclic transition state possesses keto-enol tautomerism.
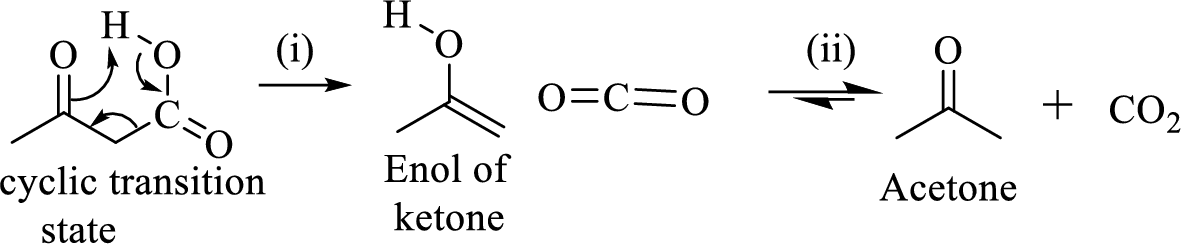
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In an attempt to synthesize compound C through a two-step process, a chemist discovered after
completing the first step that they had inadvertently produced two distinct compounds, A and B.
Upon examining the infrared spectroscopy (IR) results, it was observed that both A and B exhibited
peaks indicative of a ketone and an ester group. Please provide the molecular structures of A and B.
OEt
NaOEt
ΕΙΟ
A
B
In a chemical experiment, they noticed that both components, A and B, from a combined
sample turned into a new compound, C, during the following stage. The task is to
determine what compound C looks like and explain how compound A or B changes into
compound C through a reaction. Compound C should be the primary molecule
containing carbon created in this process, not just a by-product.
A
B
H3O+, H₂O, A
Mechanism =
с
γ-Butyrolactone (C4H6O2, GBL) is a biologically inactive compound that is converted to the biologically active recreational drug GHB (Section 19.5) by a lactonase enzyme in the body. Since γ-butyrolactone is more fat soluble than GHB, it is more readily absorbed by tissues and thus produces a faster onset of physiological symptoms. γ-Butyrolactone shows an absorption in its IR spectrum at 1770 cm−1 and the following 1H NMR spectral data: 2.28 (multiplet, 2 H), 2.48 (triplet, 2 H), and 4.35 (triplet, 2 H) ppm. What is the structure of γ-butyrolactone?
Draw the structural formula of the product of (a) p-methoxybenzaldehyde with each of the following, then do it for (b) 1-phenyl-1-pentanone for each of the following:
Chapter 17 Solutions
Organic Chemistry
Ch. 17.2 - Prob. 17.1PCh. 17.4 - Which is the stronger acid in each pair?Ch. 17.4 - Prob. 17.3PCh. 17.7 - Prob. 17.4PCh. 17.8 - Prob. 17.5PCh. 17.8 - Prob. AQCh. 17.8 - Prob. BQCh. 17.8 - Prob. CQCh. 17.8 - Permethrin and Bifenthrin Pyrethrin is a natural...Ch. 17.9 - Prob. 17.6P
Ch. 17 - Write the IUPAC name of each compound, showing...Ch. 17 - Prob. 17.8PCh. 17 - Prob. 17.9PCh. 17 - Prob. 17.10PCh. 17 - Prob. 17.11PCh. 17 - Prob. 17.12PCh. 17 - Prob. 17.13PCh. 17 - On a cyclohexane ring, an axial carboxyl group has...Ch. 17 - Prob. 17.15PCh. 17 - Prob. 17.16PCh. 17 - Prob. 17.17PCh. 17 - Complete each reaction.Ch. 17 - Prob. 17.19PCh. 17 - Prob. 17.20PCh. 17 - Prob. 17.21PCh. 17 - Show the reagents and experimental conditions...Ch. 17 - Prob. 17.23PCh. 17 - Prob. 17.24PCh. 17 - Prob. 17.25PCh. 17 - In each set, assign the acid its appropriate pKa.Ch. 17 - Low-molecular-weight dicarboxylic acids normally...Ch. 17 - Complete the following acid-base reactions. (a)...Ch. 17 - Prob. 17.29PCh. 17 - Prob. 17.30PCh. 17 - Excess ascorbic acid is excreted in the urine, the...Ch. 17 - Give the expected organic product when...Ch. 17 - Show how to convert trans-3-phenyl-2-propenoic...Ch. 17 - Show how to convert 3-oxobutanoic acid...Ch. 17 - Prob. 17.35PCh. 17 - Prob. 17.36PCh. 17 - Prob. 17.37PCh. 17 - When 4-hydroxybutanoic acid is treated with an...Ch. 17 - Fischer esterification cannot be used to prepare...Ch. 17 - Draw the product formed on thermal decarboxylation...Ch. 17 - Prob. 17.41PCh. 17 - Show how cyclohexanecarboxylic acid could be...Ch. 17 - Prob. 17.43PCh. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - Write the products of the following sequences of...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Prob. 17.51PCh. 17 - Complete the following Fischer esterification...Ch. 17 - Prob. 17.53P
Knowledge Booster
Similar questions
- Given that C6H11COOH has a pKa = 4.8 and C6H11N+H3 has a pKa = 10.7, (a) What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer? (b) What pH would you make the water layer to cause the carboxylic acid to dissolve in the ether layer and the amine to dissolve in the water layer?arrow_forwardDescribe how the addition of an isoxazolidinone to a substrate directs the stereochemistry of a reaction.arrow_forwardBisphenol A is widely used as a building block in polymer synthesis and is found in the polycarbonate hard plastics of reusable drink containers, DVDs, cell phones, and other consumer goods. Bisphenol A is reported to have estrogenic activity, and its widespread occurrence in our environment is a potential concern. Describe one or two biochemical experiments that could be done to compare the activity of bisphenol A with that of its estradiol, its structural relative.arrow_forward
- 2 4. Write the metabolism pathway for the following molecule via epoxidation of the aromatic ring and nucleophilic attack. CH3 NH₂ p-chloroamphetamine 2arrow_forwardDraw the structure of oxidised anthraquinone-2-sulfonate and the reduced structure of anthraquinone-2-sulfonate.arrow_forwardwrite the mechanism for a decarboxylation and state the structural features necessary for a decarboxylation.arrow_forward
- Define α,β-Unsaturated Carbonyl Compounds ?arrow_forwarddescribe how steric and electronic effects influence the postion of equilibrium when the electrophilic center of an aldehyde or ketone is under nucleophilic attack.arrow_forwardDescribe the mechanism and name the organic products of hydrolysis of 2,3-epoxybutane in dilute acid.arrow_forward
- Chemistry v Q8 Ketones can be obtained in one step by the Oxidation of primary alcohols Hydrolysis of esters Oxidation of secondary alcohols Reduction of acid chlorides Chemistryarrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l)CH3CH2CH2CO2H(l)+CH2CH3OH(l)⟶H+CH3CH2CH2CO2CH2CH3(l)+H2O(l) A chemist ran the reaction and obtained 5.40 g of ethyl butyrate. What was the percent yield, The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.45g of butanoic acid and excess ethanol?arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Part A Given 7.30 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. Part B A chemist ran the reaction and obtained 5.95 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. Part C The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 7.30 gg of…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning