
Concept explainers
(a)
Interpretation: The given esterification reaction equation has to completed.
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology. Esters are formed when an alcohol reacts with a
Chemically esters can be prepared by the reaction of an alcohol with an organic acid.
Esters can be simply represented as,
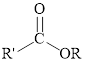
Ester formation can be represented as follows,
(b)
Interpretation: The given esterification reaction equation has to completed.
Concept introduction:
Esters encompass a large family of organic compound with broad application in various fields of science and technology. Esters are formed when an alcohol reacts with a carboxylic acid.
Chemically esters can be prepared by the reaction of an alcohol with an organic acid.
Esters can be simply represented as,

Ester formation can be represented as follows,
The reaction of –OH and –COOH on same molecule produces a cyclic ester.
Trending nowThis is a popular solution!

Chapter 17 Solutions
Organic Chemistry
- Why is it safe for us to consume foods like vinegar that contain acetic acids?arrow_forwardList the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardLabel each of the following structures as a hemiacetal, hemiketal, acetal, ketal, or none of these: a. c. b.arrow_forward
- THC is the active component in marijuana, and ethanol is the alcohol in alcoholic beverages. Explain why drug screenings are able to detect the presence of THC but not ethanol weeks after these substances have been introduced into the body.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l)CH3CH2CH2CO2H(l)+CH2CH3OH(l)⟶H+CH3CH2CH2CO2CH2CH3(l)+H2O(l) A chemist ran the reaction and obtained 5.40 g of ethyl butyrate. What was the percent yield, The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.45g of butanoic acid and excess ethanol?arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Part A Given 7.30 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. Part B A chemist ran the reaction and obtained 5.95 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. Part C The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 7.30 gg of…arrow_forward
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.45 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. A chemist ran the reaction and obtained 5.50 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 8.45 gg of butanoic acid and excess…arrow_forwardExplain in detail the structure of Lactic Acid.arrow_forwardGive an acceptable name for each aldehyde.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
