
(a)
Interpretation:
The product has to be written for the reaction involving excess
Concept Introduction:
Esters can be simply represented as,
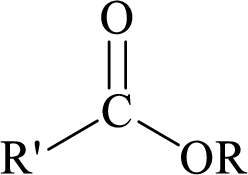
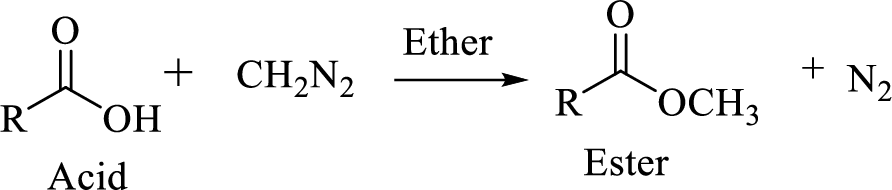
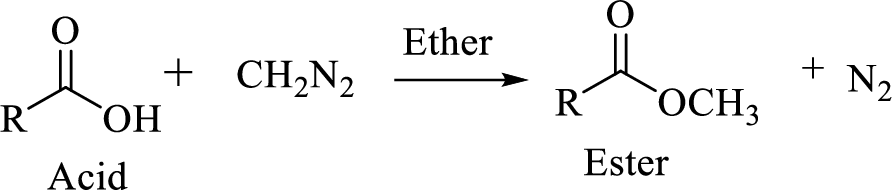
Carboxylic acids can be converted to their methyl esters by adding an ether/alcoholic solution of diazomethane.
(b)
Interpretation:
The product has to be written for the reaction involving excess
Concept Introduction:
Esters can be simply represented as,
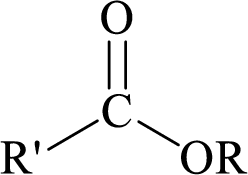
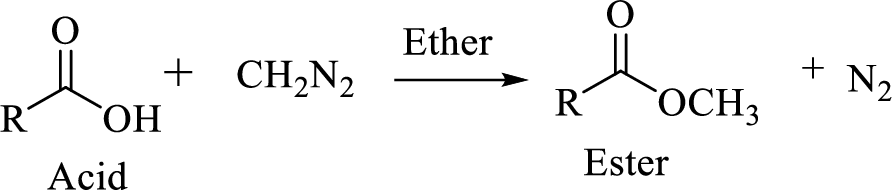
Carboxylic acids can be converted to their methyl esters by adding an ether/alcoholic solution of diazomethane.
(c)
Interpretation:
The product has to be written for the reaction involving excess
Concept Introduction:
Esters can be simply represented as,
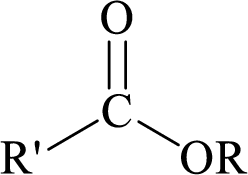
Carboxylic acids can be converted to their methyl esters by adding an ether/alcoholic solution of diazomethane.
Trending nowThis is a popular solution!

Chapter 17 Solutions
Organic Chemistry
- Which of the isomeric C4H₁0O alcohols can be prepared by hydrogenation of aldehydes? Which can be prepared by hydrogenation of ketones? Which cannot be prepared by hydrogenation of a carbonyl compound?arrow_forwardThe acid-catalyzed dehydration of 2,3-dimethyl-3-pentanol yields three alkene products. What are the names of the three alkenes? Which of the three alkenes is the major product?arrow_forwardWhen trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone i. Write the equation for the reaction between trans-2-chloro-1-cyclohexanol and the base to yield the cyclohexene oxide. ii. Why doesn’t the cis isomer yield the oxide?. iii. Write the mechanism for each of the two reactions.arrow_forward
- Suggest a simple chemical test that will differentiate between the following pairs of compounds. Write equations for the reactions involved. ▪ phenol and isopentyl alcohol ▪ tert-butyl alcohol and isobutyl alcohol ▪ neopentyl alcohol and ether ▪ sec-butyl alcohol and neopentyl alcohol ▪ propene and 2-butanolarrow_forwardIn an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H10O) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCl) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardWhen trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanoneWrite the equation for the reaction between trans-2-chloro-1-cyclohexanol and the base to yield the cyclohexene oxide Why doesn’t the cis isomer yield the oxide?Write the mechanism for each of the two reactions. .arrow_forward
- When trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone A.Write the equation for the reaction between trans-2-chloro-1-cyclohexanol and the base to yield the cyclohexene oxide B.Why doesn’t the cis isomer yield the oxide? C.Write the mechanism for each of the two reactions.arrow_forwardPropane can be used as a starting material for either propanal or propanone. Explain how this is possible. Provide a chemical equation for the reactions.arrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forward
- Hydrocarbon X has the formula C6H12.X reacts with one molar equivalent of hydrogen in the presence of a palladium catalyst to form a product having 12 primary hydrogens.Treatment of X with ozone followed by zinc in aqueous acid gives a mixture two aldehydes.What is the structure of X?arrow_forwardA compound with formula C7H12O is treated with sodium borohydride in methanol to yield 2,2-dimethylcylopentanol. Write a reaction scheme showing the structures of the reactant, the reagents, and the product. Will the product be optically active? Explain.arrow_forwardGive the structural formulae and name the functional groups of the following compounds. (a) 3-chlorobut-1-ene Name the functional group: (b) butanedioic acid Name the functional group: (c) propanamide Name the functional group: (d) 3-methylbutanal Name the functional group:arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
