
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 17, Problem 17.11P
Interpretation Introduction
Interpretation: The structural formula for monopotassium oxalate has to be drawn.
Concept introduction:
Carboxylic acids contain a carbonyl attached to a hydroxyl group as shown below,
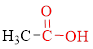
The Structural formula of a compound shows how the atoms are arranged in a molecule and, in particular, shows which
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please help I only have an hour left!!
please help I only have an hour left!
Manganese: [Ar]4s2 3d5. Mn in its +7 oxidation state (MnO4-) has a d0 configuration. Explain this situation if there are only 5 d electrons, it can lose 7 electrons to acquire the d0 configuration.
Chapter 17 Solutions
Organic Chemistry
Ch. 17.2 - Prob. 17.1PCh. 17.4 - Which is the stronger acid in each pair?Ch. 17.4 - Prob. 17.3PCh. 17.7 - Prob. 17.4PCh. 17.8 - Prob. 17.5PCh. 17.8 - Prob. AQCh. 17.8 - Prob. BQCh. 17.8 - Prob. CQCh. 17.8 - Permethrin and Bifenthrin Pyrethrin is a natural...Ch. 17.9 - Prob. 17.6P
Ch. 17 - Write the IUPAC name of each compound, showing...Ch. 17 - Prob. 17.8PCh. 17 - Prob. 17.9PCh. 17 - Prob. 17.10PCh. 17 - Prob. 17.11PCh. 17 - Prob. 17.12PCh. 17 - Prob. 17.13PCh. 17 - On a cyclohexane ring, an axial carboxyl group has...Ch. 17 - Prob. 17.15PCh. 17 - Prob. 17.16PCh. 17 - Prob. 17.17PCh. 17 - Complete each reaction.Ch. 17 - Prob. 17.19PCh. 17 - Prob. 17.20PCh. 17 - Prob. 17.21PCh. 17 - Show the reagents and experimental conditions...Ch. 17 - Prob. 17.23PCh. 17 - Prob. 17.24PCh. 17 - Prob. 17.25PCh. 17 - In each set, assign the acid its appropriate pKa.Ch. 17 - Low-molecular-weight dicarboxylic acids normally...Ch. 17 - Complete the following acid-base reactions. (a)...Ch. 17 - Prob. 17.29PCh. 17 - Prob. 17.30PCh. 17 - Excess ascorbic acid is excreted in the urine, the...Ch. 17 - Give the expected organic product when...Ch. 17 - Show how to convert trans-3-phenyl-2-propenoic...Ch. 17 - Show how to convert 3-oxobutanoic acid...Ch. 17 - Prob. 17.35PCh. 17 - Prob. 17.36PCh. 17 - Prob. 17.37PCh. 17 - When 4-hydroxybutanoic acid is treated with an...Ch. 17 - Fischer esterification cannot be used to prepare...Ch. 17 - Draw the product formed on thermal decarboxylation...Ch. 17 - Prob. 17.41PCh. 17 - Show how cyclohexanecarboxylic acid could be...Ch. 17 - Prob. 17.43PCh. 17 - Prob. 17.44PCh. 17 - Prob. 17.45PCh. 17 - Write the products of the following sequences of...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Using your reaction roadmaps as a guide, show how...Ch. 17 - Prob. 17.51PCh. 17 - Complete the following Fischer esterification...Ch. 17 - Prob. 17.53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't used hand raitingarrow_forwardHCFC-22 (CHClF2) has an atmospheric lifetime of about 12 years. CFC-12 (CCl2F2) has an atmospheric lifetime of about 100 years. Why is the atmospheric lifetime of HCFC-22 so much shorter? What atmospheric chemical contributes most to the removal of HCFC-22 from the atmosphere?arrow_forwardThe elements of group 7 (Mn, Tc, Re), with a d0 configuration, form simple oxoanions, isoelectronic with the perchlorate anion, ClO4-. Explain.arrow_forward
- Based upon the IR transmission spectrum below, would you expect HCFC-22 to function as a greenhouse gas? Explain your answer. Relative Transmittance 0.9 0.75 0.6 0.45 Methane, chlorodifluoro- INFRARED SPECTRUM 3000 2000 1000 Wavenumber (cm-1) NIST Chemistry WebBook (https://webbook.nist.gov/chemistry)arrow_forwardCheck if MnO4- and ClO4- are isoelectronic.arrow_forwardWhy were CFCs banned? How do HCFCs differ from CFCs in structure and reactivity?arrow_forward
- Nonearrow_forwardLos elementos del grupo 7 (VIIb Mn, Tc, Re) con configuración d1 forman oxoaniones MnO42- que son isomorfos e isoelectrónicos al ion sulfato SO42-. Estos oxoaniones tienen geometría tetraédrica. ¿Es esto correcto?arrow_forwardQuestion 5 of 14 Identify a correct comparison between MHC class I and class II molecules. O MHC class I molecules present peptides generated by the destruction of intracellular cytosolic proteins, whereas class Il molecules present peptides derived through phagocytosis. O The structural features of the peptide-binding groove are provided by a and B chains for class I molecules and by a; and a subdomains for class Il molecules. O Both MHC class I and class II molecules are dissimilar in their three-dimensional structures but similar in composition and the types of peptides they can bind. O The peptide-binding groove of MHC class I molecules contains a single pocket for binding peptides, whereas the groove of class II molecules contains two pockets. Question 6 of 14 Identify the similarity between MHC class I and class II molecules. O Both molecules contain two transmembrane subunits each. O Both molecules possess promiscuous binding specificity. O Both molecules accommodate peptides…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning