
(a)
Interpretation: The shape of the hybridization
Concept Introduction: In chemistry, hybridization combines two atomic orbitals to create a brand-new category of hybridized orbitals. The molecular morphologies can be well explained by the VSEPR hypothesis.
(a)
Answer to Problem 24LC
The shape of the hybridization
Explanation of Solution
Molecular bonding and shape information are both revealed by orbital hybridization.
The carbon atom with
Hybridization plays a vital role in describing double covalent bonds.
Consider a simple molecule ethene where there is a double bond between carbon and carbon and a single bond between carbon and hydrogen.
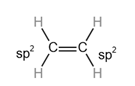
The hybridization of
Each hybrid orbital is separated by
The shape of
(b)
Interpretation: The shape of the hybridization
Concept Introduction: In chemistry, hybridization combines two atomic orbitals to create a brand-new category of hybridized orbitals. The molecular morphologies can be well explained by the VSEPR hypothesis.
(b)
Answer to Problem 24LC
The shape of the hybridization
Explanation of Solution
Molecular bonding and shape information are both revealed by orbital hybridization.
The carbon atom with
Consider a simple molecule methane where there is carbon in the center and there are four single bonds between carbon and hydrogen.
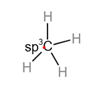
The hybridization of
Each hybrid orbital is separated by
The shape of
(c)
Interpretation: The shape of the hybridization
Concept Introduction: In chemistry, hybridization combines two atomic orbitals to create a brand-new category of hybridized orbitals. The molecular morphologies can be well explained by the VSEPR hypothesis.
(c)
Answer to Problem 24LC
The shape of the hybridization
Explanation of Solution
Molecular bonding and shape information are both revealed by orbital hybridization.
The carbon atom with
Hybridization plays a vital role in describing triple covalent bonds.
Consider a single molecule of acetylene where there is a triple bond between carbon and carbon and a single bond between carbon and hydrogen.
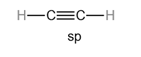
The hybridization of
Each hybrid orbital is separated by
The shape of
Chapter 8 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





