
(a)
Interpretation: The electron dot structure for
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
According to the octet rule, all the atoms in a molecule are bonded to other atoms in such a way that they have complete octets (8 electrons in valence shell). There are some exceptions and some molecule does not obey the octet rule. This is due to the odd number of electrons, the number of electrons more than 8, and the number of electrons less than 8.
(a)
Explanation of Solution
The given molecule is
. Here, the number of the valence electron in Be is 2 and that in F is 7. The electron dot structure can be represented as follows:
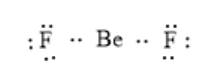
Here, Be does not have a complete octet; thus, the octet rule is not obeyed.
(b)
Interpretation: The electron dot structure for
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
According to the octet rule, all the atoms in a molecule are bonded to other atoms in such a way that they have complete octets (8 electrons in valence shell). There are some exceptions and some molecule does not obey the octet rule. This is due to the odd number of electrons, the number of electrons more than 8, and the number of electrons less than 8.
(b)
Explanation of Solution
The given molecule is
. Here, the number of valence electrons in Si is 4 and that in F is 7. The electron dot structure can be represented as follows:
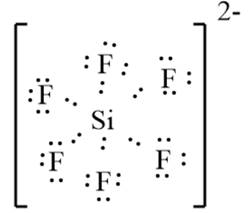
Since there are 4 valence electrons on the Si atom it must gain 2 electrons to bond 6 F atoms. Thus, there will be an overall -2 charge. Here, the Si atom has an extended configuration as there are more than 8 electrons.
(c)
Interpretation: The electron dot structure for
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
According to the octet rule, all the atoms in a molecule are bonded to other atoms in such a way that they have complete octets (8 electrons in valence shell). There are some exceptions and some molecule does not obey the octet rule. This is due to the odd number of electrons, the number of electrons more than 8, and the number of electrons less than 8.
(c)
Explanation of Solution
The given molecule is
. Here, the Cl atom has 7 valence electrons and the O atom has 6 valence electrons. The arrangement of electrons around atoms takes place according to the following electron dot structure:
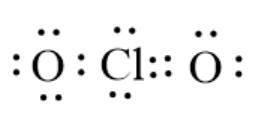
Here, the Cl atom does not have 8 electrons in the valence shell; thus, it does not follow the octet rule. Here, the number of electrons in the valence shell is more than 8; thus, it has an extended configuration.
(d)
Interpretation: The electron dot structure for
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
According to the octet rule, all the atoms in a molecule are bonded to other atoms in such a way that they have complete octets (8 electrons in valence shell). There are some exceptions and some molecule does not obey the octet rule. This is due to the odd number of electrons, the number of electrons more than 8, and the number of electrons less than 8.
(d)
Explanation of Solution
The given molecule is
. Here, the number of valence electrons in B is 3, and that in F is 7. The electron dot structure can be represented as follows:
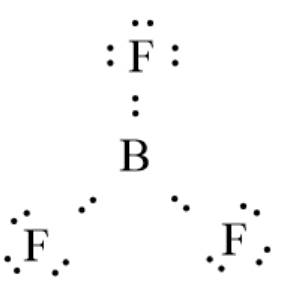
There are 6 electrons in the valence shell thus, it has less than 8 electrons. Here, boron does not have a complete octet thus, the octet rule is not obeyed.
(e)
Interpretation: The electron dot structure for
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
According to the octet rule, all the atoms in a molecule are bonded to other atoms in such a way that they have complete octets (8 electrons in valence shell). There are some exceptions and some molecule does not obey the octet rule. This is due to the odd number of electrons, the number of electrons more than 8, and the number of electrons less than 8.
(e)
Explanation of Solution
The given molecule is
. Here, the number of the valence electron in Xe is 8 and that in F is 7. The electron dot structure can be represented as follows:
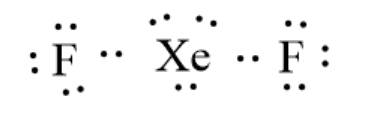
Now, Xe has more than 8 electrons thus, the octet rule is not obeyed.
Chapter 8 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





