
Interpretation: The electron dot structures of carbon monoxide and carbon dioxide need to be drawn. The structural differences between the two molecules need to be explained.
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
Explanation of Solution
The carbon monoxide molecule contains carbon and oxygen atoms having triple bonds between them. Here, the carbon atom has 4 valence electrons and the oxygen atom has 6 valence electrons. The arrangement of total valence electrons around the atoms takes place in such a way that there are 3 bonding pairs of electrons and 2 non-bonding pairs of electrons.
The electron dot structure is represented as follows:
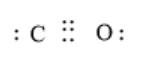
The carbon dioxide molecule also contains carbon and oxygen atoms, but here one carbon atom is bonded with two oxygen atoms. The arrangement of the total number of valence electrons is such that there are 4 bonding pairs of electrons and 4 non-bonding pairs of electrons.
The electron dot structure is represented as follows:
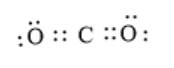
The structural difference between carbon monoxide and carbon dioxide is that there are no lone pairs of electrons on the C atom in carbon dioxide but there is one lone pair of electrons on the carbon atom in carbon monoxide. Also, the number of lone pairs of electrons on the O atom of carbon monoxide is 1 but, in carbon dioxide, both oxygen atoms have two lone pairs of electrons each.
Chapter 8 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





