
(a)
Interpretation:
Electron dot structure of
Concept Interpretation:
Diagrams that show the
(a)
Answer to Problem 58A
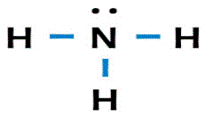
One nitrogen (
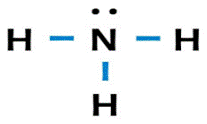
Explanation of Solution
Nitrogen has a total of
valence electrons and hydrogen has a total of one valence electron. The Lewis structure is as follows:
(b)
Interpretation:
Electron dot structure of
Concept Interpretation:
Diagrams that show the chemical bonding between atoms in a molecule are known as Lewis’s dot structures, also known as electron dot structures.
(b)
Answer to Problem 58A
One Bromine (

Explanation of Solution
The number of valence electrons in bromine is seven and chlorine contains

(c)
Interpretation:
Electron dot structure of
Concept Interpretation:
Diagrams that show the chemical bonding between atoms in a molecule are known as Lewis’s dot structures, also known as electron dot structures.
(c)
Answer to Problem 58A
Two sulfur (

Explanation of Solution
Two sulfur (

(d)
Interpretation:
Electron dot structure of
Concept Interpretation:
Diagrams that show the chemical bonding between atoms in a molecule are known as Lewis’s dot structures, also known as electron dot structures.
(d)
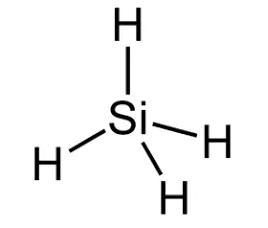
Answer to Problem 58A
The center atom is the silicon which contains four valance electrons while the hydrogen atoms have one valance electron.
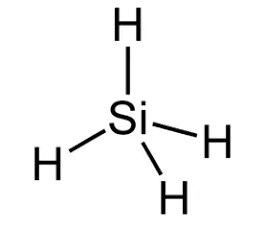
Explanation of Solution
In the SiH4 molecule, the core silicon atom has four valence electrons and the hydrogen atom has one.
The SiH4 molecule has no formal charge.
Chapter 8 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





