
(a)
Interpretation: The geometry around the central atom in the given molecule needs to be determined.
Concept Introduction: VSEPR theory is used to predict the molecular shape with the help of electron pairs around the central atoms of the molecule. It is based on the assumption that the shape of the molecule is such that there is minimum electronic repulsion between the valence shell and the atom.
(a)
Explanation of Solution
According to VSEPR theory, the following table shows the relation between the total number of bonding and non-bonding pair of electrons and the geometry of a molecule.
| Total number of bonding and non-bonding pairs | Hybridization | Electron pair geometry | Shape | ||||
| No lone pairs | 1 | 2 | 3 | 4 | |||
| 6 | Octahedral | Octahedral | Square pyramidal | Square planar | T-shaped | Linear | |
| 5 | Trigonal pyramidal | Trigonal pyramidal | Sawhorse | T-shared | Linear | ||
| 4 | Tetrahedral | Tetrahedral | Trigonal planar | Bent | |||
| 3 | Trigonal planar | Trigonal planar | Bent | ||||
| 2 | Linear | Linear | |||||
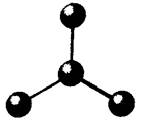
The given molecule is as follows:
Here, central atom is bonded with three atoms via single bond. Also, there is no lone pair of electrons on central atom. The hybridization of central metal atom is
(b)
Interpretation: The geometry around the central atom in the given molecule needs to be determined.
Concept Introduction: VSEPR theory is used to predict the molecular shape with the help of electron pairs around the central atoms of the molecule. It is based on the assumption that the shape of the molecule is such that there is minimum electronic repulsion between the valence shell and the atom.
(b)
Explanation of Solution
According to VSEPR theory, the following table shows the relation between the total number of bonding and non-bonding pair of electrons and the geometry of a molecule.
| Total number of bonding and non-bonding pairs | Hybridization | Electron pair geometry | Shape | ||||
| No lone pairs | 1 | 2 | 3 | 4 | |||
| 6 | Octahedral | Octahedral | Square pyramidal | Square planar | T-shaped | Linear | |
| 5 | Trigonal pyramidal | Trigonal pyramidal | Sawhorse | T-shared | Linear | ||
| 4 | Tetrahedral | Tetrahedral | Trigonal planar | Bent | |||
| 3 | Trigonal planar | Trigonal planar | Bent | ||||
| 2 | Linear | Linear | |||||
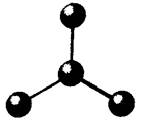
The given molecule is as follows:
Here, the central atom is bonded with two atoms via a single bond. Also, there is no lone pair of electrons on the central atom. The hybridization of the central metal atom is
(c)
Interpretation: The geometry around the central atom in the given molecule needs to be determined.
Concept Introduction: VSEPR theory is used to predict the molecular shape with the help of electron pairs around the central atoms of the molecule. It is based on the assumption that the shape of the molecule is such that there is minimum electronic repulsion between the valence shell and the atom.
(c)
Explanation of Solution
According to VSEPR theory, the following table shows the relation between the total number of bonding and non-bonding pair of electrons and the geometry of a molecule.
| Total number of bonding and non-bonding pairs | Hybridization | Electron pair geometry | Shape | ||||
| No lone pairs | 1 | 2 | 3 | 4 | |||
| 6 | Octahedral | Octahedral | Square pyramidal | Square planar | T-shaped | Linear | |
| 5 | Trigonal pyramidal | Trigonal pyramidal | Sawhorse | T-shared | Linear | ||
| 4 | Tetrahedral | Tetrahedral | Trigonal planar | Bent | |||
| 3 | Trigonal planar | Trigonal planar | Bent | ||||
| 2 | Linear | Linear | |||||
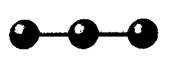
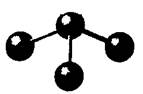
The given molecule is as follows:
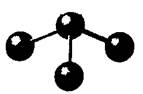
Here, the central atom is bonded with three atoms via a single bond. The geometry is bent which indicates one lone pair of electrons on the central atom. Due to one lone pair of electrons and three bond pairs of electrons, the central atom has
(d)
Interpretation: The geometry around the central atom in the given molecule needs to be determined.

Concept Introduction: VSEPR theory is used to predict the molecular shape with the help of electron pairs around the central atoms of the molecule. It is based on the assumption that the shape of the molecule is such that there is minimum electronic repulsion between the valence shell and the atom.
(d)
Explanation of Solution
According to VSEPR theory, the following table shows the relation between the total number of bonding and non-bonding pair of electrons and the geometry of a molecule.
| Total number of bonding and non-bonding pairs | Hybridization | Electron pair geometry | Shape | ||||
| No lone pairs | 1 | 2 | 3 | 4 | |||
| 6 | Octahedral | Octahedral | Square pyramidal | Square planar | T-shaped | Linear | |
| 5 | Trigonal pyramidal | Trigonal pyramidal | Sawhorse | T-shared | Linear | ||
| 4 | Tetrahedral | Tetrahedral | Trigonal planar | Bent | |||
| 3 | Trigonal planar | Trigonal planar | Bent | ||||
| 2 | Linear | Linear | |||||
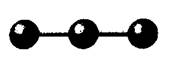
The given molecule is as follows:
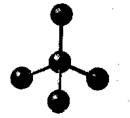
Here, the central atom is bonded with three atoms via a single bond. Also, there is no lone pair of electrons on the central atom. The hybridization of the central metal atom is
Chapter 8 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





