
(a)
Interpretation: The electron dot structure of ethanoic acid needs to be drawn.
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
(a)
Explanation of Solution
The molecular formula of ethanoic acid is
The number of valence electrons in C, H, and O atoms is 4, 1, and 6 respectively. Thus, the total number of valence electrons in ethanoic acid will be:
The total number of valence electrons is distributed among atoms such that there are 8 bonding-pair of electrons and 4 lone-pair of electrons.
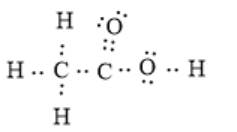
The electron dot structure can be represented as follows:
(b)
Interpretation: Whether the bonding between each oxygen atom and carbon is the same or not needs to be explained.
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
(b)
Explanation of Solution
In the given molecule, there are two carbon and two oxygen atoms. The two carbon atoms are bonded together via a single covalent bond. Here, one oxygen atom is bonded with a carbon atom via one double bond, and the other oxygen atom is bonded via a single bond. The oxygen atom singly bonded with the carbon atom is also bonded with one hydrogen atom.
Therefore, the bonding between each oxygen atom and the carbon atom is not the same.
(c)
Interpretation: Whether the bonding between the carbon and each oxygen atom is polar or nonpolar needs to be explained.
Concept Introduction: For a covalent bond to be polar, the difference in electronegativity should be between 0.5 to 2.0. Here, one atom has more electronegativity than another; thus, the electronegative atom has a partial negative charge and the less electronegative atom has a partial positive charge.
(c)
Explanation of Solution
A covalent bond is said to be polar in nature if the difference between the electronegativity value of two atoms is between 0.5 to 2.0. Here, the bond formed between carbon and oxygen needs to be analyzed. The electronegativity value of carbon and oxygen is 2.55 and 3.44 respectively. The difference in electronegativity can be calculated as follows:
Since the calculated value is between 0.5 to 2.0, the carbon-oxygen bond will be polar in nature.
(d)
Interpretation: Whether ethanoic acid is a polar molecule or not needs to be determined.
Concept Introduction: A molecule is said to be polar if there are polar bonds and the molecule has some net dipole moment.
(d)
Explanation of Solution
A molecule is said to be polar in nature if it has polar bonds and it has an overall dipole moment.
The structure of ethanoic acid is as follows:
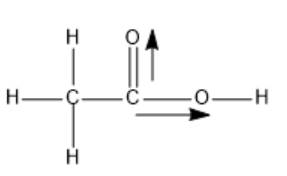
Here, dipole moments of two C-O bonds are not get canceled out; thus, the overall molecule is polar in nature.
Chapter 8 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





