
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 11P
The following data describe the catalysis of cleavage of peptide bonds small peptides by the enzyme elastase.
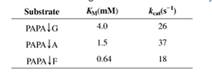
The arrow indicates the peptide bond cleaved each case.
a. If a mixture of these three substrates was presented to elastase with the concentration of each peptide equal to 0.5 mM, which would be digested most rapidly? Which most slowly? (Assume enzyme present in excess.)
b. On basis of these data, suggest what features of amino acid sequence dictate the specificity of proteolytic cleavage by elastase.
c. Elastase is closely related to chymotrypsin. Suggest two kinds of amino acid residues you might expect to find in or near the active site.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The following data describe the catalysis of cleavage of peptide bonds in small peptides by the enzyme
UTSASE (the arrow indicates the peptide bond cleaved in each case).
Substrate
Km(mM)
kcat(s)
PAPALG
4.0
26
РАPАLA
1.5
37
РАРАLF
0.64
18
Based on the above data shown for UTSAse what features of amino acid sequence dictate the specificity of
the proteolytic cleavage?
A. Large hydrophilic R-groups
B. Large hydrophobic R-groups
C. Neutral R-groups
D. Small hydrophilic R-groups
E. Large hydrophobic R-groups
F. Negatively charged R-groups
G. Positively charged R-groups
The following data describe the catalysis of cleavage of peptide bonds insmall peptides by the enzyme elastase. The arrow indicates the peptide bond cleaved in each case.(a) If a mixture of these three substrates was presented to elastase withthe concentration of each peptide equal to 0.5 mM, which would bedigested most rapidly? Which most slowly? (Assume enzyme is presentin excess.)(b) On the basis of these data, suggest what features of amino acid sequence dictate the specificity of proteolytic cleavage by elastase.(c) Elastase is closely related to chymotrypsin. Suggest two kinds of aminoacid residues you might expect to find in or near the active site.
The ESI-MS spectrum in positive ionization mode for lysozyme is obtained.
a. What is the molecular weight of the protein to 5 significant figures based on the two highlighted ion species?
b. What is the charge of the peaks at 1101.5 and 1789.2.
Chapter 8 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 8 - Prob. 1PCh. 8 - The enzyme urease catalyzes the hydrolysis of urea...Ch. 8 - An enzyme contains an active site aspartic acid...Ch. 8 - The folding and unfolding rate constants for a...Ch. 8 - In some reactions, in which a protein molecule is...Ch. 8 - Would you expect an “enzyme” designed to bind to...Ch. 8 - The initial rate for an enzyme-catalyzed reaction...Ch. 8 - a. If the total enzyme concentration in Problem 7...Ch. 8 - Prob. 9PCh. 8 - Prob. 10P
Ch. 8 - The following data describe the catalysis of...Ch. 8 - At 37 oC, the serine protease subtilisin has kcat...Ch. 8 - The accompanying figure shows three...Ch. 8 - The steady-state kinetics of an enzyme are studied...Ch. 8 - The same enzyme as in Problem 14 is studied in the...Ch. 8 - Enalapril is an anti-hypertension “pro-drug"...Ch. 8 - Initial rate data for an enzyme that obeys...Ch. 8 - Prob. 18PCh. 8 - Suggest the effects of each of the following...Ch. 8 - The inhibitory effect of an uncompetitive...Ch. 8 - Prob. 21PCh. 8 - Prob. 22PCh. 8 - Prob. 23PCh. 8 - In kinetics experiments, the hydrolysis of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Partial hydrolysis of a protein yielded a number of polypeptides. One of them was purified. Deduce the sequence of amino acids in this polypeptide from the following information: (a) Complete acid hydrolysis yielded ala + arg + 2ser + lys + phe + met + trp + pro (b) Treatment with fluorodinitrobenzene (FDNB, the Sanger reagent) followed by complete acid hydrolysis yielded dinitrophenylalanine (DNP-ala) and a dinitrophenyllysine (e-DNP-lys) as the only DNP derivatives. (c) Neither carboxypeptidase A nor carboxypeptidase B released a C-terminal amino acid. (d) Treatment with cyanogen bromide (CNBr) yielded two peptides: One contained ser + trp + pro. The other contained all the remaining amino acids (including the second ser). (e) Treatment with chymotrypsin yielded three peptides. One contained only ser + pro. Another contained only met + trp. The third contained phe + lys + ser + arg + ala. (f) Treatment with trypsin yielded three peptides. One contained only ala + arg. Another…arrow_forwardBovine pancreatic trypsin inhibitor (BPTI; as shown) contains six cysteine residues that form three disulfide bonds in the native structure of BPTI. Suppose BPTI is reduced and unfolded in urea (as illustrated for RNase A as shown). If the reduced unfolded protein were oxidized prior to the removal of the urea, what fraction of the resulting mixture would you expect to possess native disulfide bonds?arrow_forwardSuppose that there is a protein consisting of two polypeptide chains with the given sequences in the picture. Answer the following questions. a. What digestion or cleavage agent(s) should a biochemist use to determine the sequence of subunit α? Explain why. b. What digestion or cleavage agent(s) should a biochemist use to determine the sequence of subunit β? Explain why. c. Identify the resulting peptide fragment(s) of subunit α. Identify the resulting peptide fragment(s) of subunit β.arrow_forward
- When protein X is subjected to dimethylsuberimidate and SDS electrophoresis a 50 kDa band is seen; when the protein X is subjected to SDS electrophoresis without DMS, two bands of 20 kDa and 10 kDa are seen. How many different oligomeric structures of the native protein X are possible, being consistent with these observed results? one two three none of the above which is the answrr? and explainarrow_forwardThe following steps were performed using enzyme cleavage of a peptide to determine its amino acid sequence. Step 1. FDNB yield DNB-Gly Step 2. Treatment with trypsin yield 3 fragments: Tyr-Leu-Asp-Arg; Gly-Ser-Ala-Lys; Trp-Gly-Ser-Met Step 3. Treatment with pepsin gave the same 3 peptide fragments. What is the sequence of the peptide?arrow_forwarddescribe a detailed experimental procedure for the chemical synthesis of proteins with the α-ketoacid hydroxylamine (KAHA ligation), using (S)-5-oxaproline (Opr) as a key building block. This protocol comprises two main parts: (i) the synthesis of peptide fragments by standard fluorenylmethoxycarbonyl (Fmoc) chemistry and (ii) the KAHA ligation between fragments containing Opr and a C-terminal peptide α-ketoacid.arrow_forward
- Considering the chemical characteristics of the amino acids valine and glutamic acid (see Figure 5.14), propose a possible explanation for the dramatic effect on protein function that occurs when valine is substituted for glutamic acid.arrow_forwardThe trypsin enzyme is able to hydrolyze a peptide substate at the carboxyl side of an Arg or Lys residue. However, such a reaction can be also influenced by the amino acid residue that follows Arg or Lys. Given the Michaelis-Menton plots obtained for two substrates (substrate A: Ser-Val- Arg-Pro; substrate B: Ser-Val-Arg-Phe), the Km of the enzyme is higher for: 20 Substrate A Assume that these curves do not reach the same limit. 15 Substrate E 0.001 0.002 0.003 (Substrate) (molar) Inconclusive Substrate B Both Substrate A and B Intitial velocity (micromoles/literisecond)arrow_forwardExperimental results describing a protein's amino acid composition are useful for estimating the molecular weight (MW) of the entire protein. A quantitative amino acid analysis reveals that bovine cytochrome c contains 1% tryptophan (M, 204) by weight. Calculate the approximate molecular weight of bovine cytochrome c if there is 1 tryptophan residue. Please enter your answer with three significant figures. approximate bovine cytochrome c MW: number of threonine residues: 19.4 20.4 x10³ Bovine chymotrypsinogen has a molecular weight of 25.6 kDa. Amino acid analysis shows that this enzyme is 9% threonine (M, 119). Calculate how many threonine residues are present in a molecule of bovine chymotrypsinogen. Round your answer to the nearest whole number. Incorrect Incorrect Daarrow_forward
- you were given an unknown peptide. Explain in detail how you would determine the amino acid sequence of this peptide by means of Edman degradation. (You can do this by showing how you would determine the N-terminal amino acid of the peptide shown below in figure 2 with Edman degradation.arrow_forwardSome of the following four amino acids : alanine, arginine, histidine, aspartic acid would provide a side chain for acid-base catalysis at physiological pH (assume pK of each amino acid is equal to pK value for the free amino acid in solution). Explain for each amino acid how and why each would or would not provide the side chain residue to support acid-base catalysis at physiological pH.arrow_forwardWhen performing his experiments on protein refolding, Christian Anfinsen obtained a quite different result when reduced ribonuclease was reoxidized while it was still in 8 M urea and the preparation was then dialyzed to remove the urea. Ribonuclease reoxidized in this way had only 1% of the enzymatic activity of the native protein. Why were the outcomes so different when reduced ribonuclease was reoxidized in the presence and absence of urea?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY