
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8P
a. If the total enzyme concentration in Problem 7 was 1 nmol/L, how many molecules of substrate can a molecule of enzyme process in each minute?
b. Calculate kcat/ KM for the enzyme reaction in Problem 7. Is this a fairly efficient enzyme? (See Table 8.5.)
7. The initial rate for an enzyme-catalyzed reaction has been determined at a number of substrate concentrations. Data are as follows:
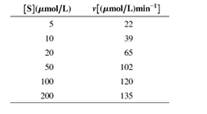
- Estimate Vmax and KM from a direct graph v versus [S]. Do you find difficulties in getting clear answers?
- Now use a Lineweaver-Burk plot to analyze the same data. Does this work better?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You will perform the protocol below for the calf intestinal alkaline phosphatase (CIP) provided. For each reaction, your final enzyme concentration should be 10 nM CIP.
Note: Enzymes purchased are typically labelled with their “units of activity” (U), as this relates to how much enzyme is needed to catalyze a reaction. The 100 nM CIP provided has approximately 3 U/mL and was diluted 1 in 1,000 from a 500 U/mL purchased enzyme.
1) Create a table (similar to the one below) to help you determine and keep track of what to add to each of the cuvettes in which your reactions will be measured. The five different concentrations of PNPP should be: 25, 50, 100, 200, 300 μM. Each reaction will be in a final volume of 1 mL and contain 10 nM alkaline phosphatase.
Concentrations of stock solutions: 1.0 mM PNPP, 100 nM calf intestinal phosphatase
Shown below are Km, and Vmax values obtained for an enzyme A which catalyze the transformation of
the following substrates. Enzyme concentration used was 0.01 M.
Km, mM
0.02
Vmax, mM/min
5.3
Substrate
1
2
1.5
13.7
3
2.6
100
4
0.1
25
0.05
62
1. Which substrate have the highest affinity for the enzyme? Explain.
2. Which will show higher efficiency of converting the substrate to the product? Show solutions and
еxplain.
Consider the given data for an enzyme-catalyzed reaction. Determine the Vm, Km and the type of
inhibition based on the given data below
Substrate concentration, uM
30
50
100
300
900
slope
y-intercept
Complete the table below (include correct units).
Experiment A
Vm
Km
Experiment A
(Initial velocity without
inhibitor, uM-min)
Type of Inhibition:
10.4
14.5
22.5
33.8
40.8
Experiment B
(Initial velocity with inhibitor,
uM-min)
5.1
7.3
13.3
25.7
37.2
Experiment B
Chapter 8 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 8 - Prob. 1PCh. 8 - The enzyme urease catalyzes the hydrolysis of urea...Ch. 8 - An enzyme contains an active site aspartic acid...Ch. 8 - The folding and unfolding rate constants for a...Ch. 8 - In some reactions, in which a protein molecule is...Ch. 8 - Would you expect an “enzyme” designed to bind to...Ch. 8 - The initial rate for an enzyme-catalyzed reaction...Ch. 8 - a. If the total enzyme concentration in Problem 7...Ch. 8 - Prob. 9PCh. 8 - Prob. 10P
Ch. 8 - The following data describe the catalysis of...Ch. 8 - At 37 oC, the serine protease subtilisin has kcat...Ch. 8 - The accompanying figure shows three...Ch. 8 - The steady-state kinetics of an enzyme are studied...Ch. 8 - The same enzyme as in Problem 14 is studied in the...Ch. 8 - Enalapril is an anti-hypertension “pro-drug"...Ch. 8 - Initial rate data for an enzyme that obeys...Ch. 8 - Prob. 18PCh. 8 - Suggest the effects of each of the following...Ch. 8 - The inhibitory effect of an uncompetitive...Ch. 8 - Prob. 21PCh. 8 - Prob. 22PCh. 8 - Prob. 23PCh. 8 - In kinetics experiments, the hydrolysis of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider an enzyme that follows standard Michaelis-Menten kinetics and has the following kinetic constants: %3| k2 = 1.5 x 10? s1 Еo 3D 1 х 104 М = 1 x 104 M a. What is the value of the maximum rate VM? b. Prepare a hand-drawn quantitative plot on graph paper (not a simple sketch nor an EXCEL-generated graph) of the enzymatic reaction rate versus substrate concentration (like Fig 3-3) using the kinetic parameters given above. Be sure to label the axes and include numeric values on the axes. C. Based upon your hand drawn saturation plot, at what substrate concentration is the enzymatic reaction rate 75% of Vm?arrow_forwardThe change in the amount of product B in a reaction which is catalyzed by Enzyme A is given in the table below. Determine the Vmax and Km of the enzyme in the absence and presence of C. Is C an inhibitor or an activator of enzyme A? Determine the type of inhibition or activation. Rate of formation of Product B in the presence of 25 mg/mL C (mM/min) Substrate Rate of formation of (mM) Product B (mM/min) 0.5 21.5 14.1 1 30.2 22.2 1.5 34.9 27.0 2.5 39.8 33.1 3.5 42.0 36.3arrow_forward(b) You are investigating the effects of several agents on the activity of alcohol dehydrogenase. The enzyme activity data are shown in the table below. Construct a [substrate] vs. activity plot and a double-reciprocal plot for this enzyme. Be sure to label all axes. Determine the Vmax and KM for AD from the graphs in each type of plot. AD activity (nM/min) AD activity + agent A (nM/min) AD activity + agent B (nM/min) [Alcohol] (nM) 0.1 14 2 0.5 50 7 8. 1.0 65 10 30 2.0 72 12 45 4.0 80 14 62 8.0 85 15 75 32.0 90 16 90arrow_forward
- Below is kinetic data obtained for an enzyme-catalyzed reaction. The enzyme concentration is fixed at 100 nM. Using a Lineweaver-Burke plot, calculate the Vmax value for this reaction. Report your answer to four significant figures in units of uM/min.arrow_forwardAlthough graphical methods are available for accurate determination of the Vmax and Km of an enzyme-catalyzed reaction, sometimes these quantities can be quickly estimated by inspecting values of V0 at increasing [S]. Estimate the Vmax and Km of the enzyme-catalyzed reaction for which the following data were obtained:arrow_forwardFor an enzyme obeying the Michaelis-Menten equation with Km = 5 µM, kcat = 10 s-¹ and a total enzyme concentration of 1 nM, Calculate Vmax. Calculate the substrate concentration at which v = 0.1 Vmax Calculate the substrate concentration at which v = 0.9 Vmax What fraction of the enzyme is bound to substrate when v = 0.9 Vmax? Sketch a graph showing the Michaelis-Menten plot. Make sure you label the axes on your plot. Label on the graph: i) the point on the graph at which S=Km ii) Vmax At low substrate concentration, v = Keat [Etot] [S] Km Circle on your graph where this equation applies. What name is used to refer to keat/Km? : Calculate keat/Km for this enzyme. How does this value compare with the fastest enzymes?arrow_forward
- Sketch and label a plot showing the enzyme's initial velocity relative to pH over the pH range 4 - 9 for the enzyme-catalyzed reaction under these two conditions: A. The substrate concentration is very, very high. B. The substrate concentration is less than the enzyme's Km.arrow_forwardThe kinetics of an enzyme were measured as a function of substrate concentration in the presence or absence of 10-4 M inhibitor. The following initial rate data were obtained: Substrate (So µM/L) 3 5 Velocity (Vo, µmol/L-min) Without inhibition 10 30 90 10.4 14.5 4.5 6.8 8.1 Is this a competitive or noncompetitive inhibition? Evaluate KM, KI, and Vmax. (HINT: use linear regression to first estimate kinetic parameters for uninhibited and then for the inhibited) With inhibition 2.1 2.9 22.5 33.8 40.5arrow_forwardAn enzyme catalyzes a reaction with a Km of 9.50 mM and a Vmax of 2.10 mM · s-1. Calculate the reaction velocity, vo, for each substrate concentration. [S] = 3.25 mM vo : mM · s-1 [S] = 9.50 mM mM · s-1 Vo :arrow_forward
- Compound A is the substrate for two enzymes, El and E2, their reaction rates, r1 and r2,Determine the Km and rmax for both enzymes, with respect to the concentration of A. Which set of data is more likely to be for El and which for E2, and why? Concentration of 0.2 0.6 1.2 3 4 5 6 8 12 15 A (mM) Reaction rate (r.) (mmol/L'min) 3.33 4.29 4.62 4.76 4.84 4.88 4.9 4.92 4.94 4.95 4.96 4.97 Reaction rate (r,) (mmol/L*min) 0.09 0.23 0.38 0.5 0.6 0.67 0.71 0.75 0.8 0.82 0.86 0.80arrow_forwardAn enzyme that follows simple Michaelis–Menten kinetics has an initial reaction velocity of 10 µmol⋅min-1 when the substrate concentration is five times greater than the KM. What is the Vmax of this enzyme in µmol⋅min−1?arrow_forwardThe Michaelis-Menten equation models the hyperbolic relationship between [S] and the initial reaction rate Vo for an enzyme-catalyzed, single-substrate reaction E + S ES → E + P. The model can be more readily understood when comparing three conditions: [S] > Km- Match each statement with the condition that it describes. Note that "rate" refers to initial velocity Vo where steady state conditions are assumed. [Etotal] refers to the total enzyme concentration and [Efree] refers to the concentration of free enzyme. [S] > Km Not true for any of these conditions Almost all active sites will [ES] is much lower than [Efree]. be filled. The rate is directly proportional to Increasing [Etotal] will increase [S]. Km: Adding more S will not increase [Efree] is equal to [ES]. the rate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Enzyme Kinetics; Author: MIT OpenCourseWare;https://www.youtube.com/watch?v=FXWZr3mscUo;License: Standard Youtube License