
Concept explainers
In kinetics experiments, the hydrolysis of the substrate sialic acid by neuraminidase appears to obey Michaelis—Menten kinetics. Neuraminidase activity is critical for viral infectivity; thus, this enzyme is the target of much work by pharmaceutical companies to develop a drug to treat influenza virus
infection. The drug "Tamiflu" is a competitive inhibitor of neuraminidase. Initial rate data collected at
pH = 6.15, 37 oC with 0.021 µM neuraminidase and 25.0 µM sialic acid gives a Lineweaver—Burk plot with a slope of 51.2 s.
a. Recall from Problem that the kcat for neuraminidase at pH = 6.15, 37 oC is 26.8 s-1. Calculate KM for the hydrolysis of sialic acid.
b. When the reactions in part (a) are repeated in the presence of 0.040 µM of Tamiflu, the slope of the Lineweaver—Burk plot is 198.8 s. Calculate the value of KI for Tamiflu.
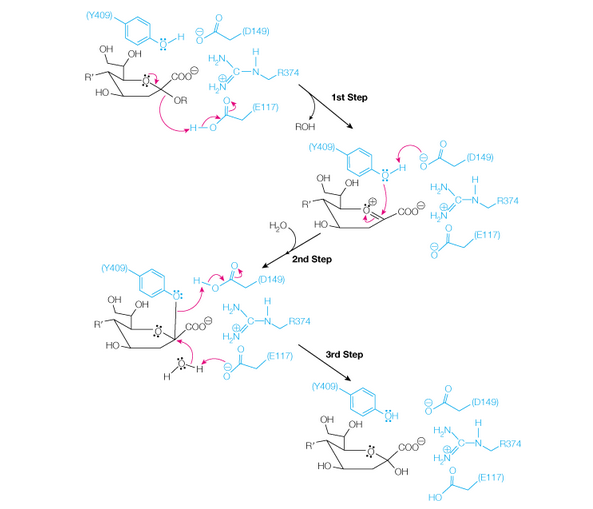
23. Shown below is a proposed mechanism for the cleavage of sialic acid by the viral enzyme
neuraminidase. The kcat for the wild-type enzyme at pH = 6.15, 37 oC is 26.8 s-1.
a. Describe the roles of the following amino acids in the catalytic mechanism: Glu117, Tyr409, and Asp149. List all of the following that apply: general acid/base catalysis (GABC), covalent catalysis, electrostatic stabilization of transition state.
b. Based on the information shown in the scheme, would you expect mutation of Glu117 to Ala to have a greater effect on KM or kcat?
c. For the R374N mutant at pH = 6.15, 37 oC, kcat is 0.020 s-1, and KM is relatively unaffected. Based on this result, it seems that R374 is more critical for catalysis than for substrate binding.
Explain how R374 stabilizes the reaction transition state more than the substrate (i.e., what feature of this reaction would explain tighter binding to the transition state vs. substrate?).
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
- Chitinase is a protein that breaks down chitin, a primary component of the cell wall in fungi, scales in fish and exoskeletons of arthropods. The activity of chitinase extracted from a plant was shown to be optimum at pH 5. You were tasked to prepare 300 mL of 150 mM buffer solution for further analysis of the extracted chitinase. REAGENTS Ka 2.5M Acetic acid Solid NaOAc•3H2O [136.08g/mol] 1.76 x 10-5 2.5M NH3 Solid NH4Cl [53.49g/mol] 5.6 x 10-10 2.5M Lactic acid Solid sodium lactate [112.06g/mol] 4.0 x 10-5 5 M HCl 5M NaOH Pls show sol'ns 1. Given the following reagents, give the moles of each component (acid & base).2. What are the mass/volume of the components needed to prepare the buffer? 3. What will the pH of the buffer be if 1mL of 5 M NaOH was added?arrow_forwardThe glucose oxidase used in this experiment has a concentration of 27 U/mg. Calculate the mass (in g) of solid glucose oxidase required to react with all the glucose in 4.00 µL of10.0- mM glucose sample assuming the reaction proceeds for 30 minutes.arrow_forwardA bacterial enzyme catalyzes the hydrolysis of maltose as shown in the reaction given below: Maltose + H2O -> 2 glucose If the reaction has a Km of 0.135 mM and a V max of 65 umol/min. What is the reaction velocity when the concentration of maltose is 1.0 mM? (Please take note of the units)arrow_forward
- Calculate the Km of the enzyme with these parameters. kcat = 130s^-1 Vo = 3.0 μMs-1 S = 10 μM Et = 0.09 µMarrow_forwardThe co-amoxiclav disc (20/10) has two drugs present, amoxicillin and clavulanate. The purpose of clavulanate is best described by which ONE of the following? Select one: A. To increase the solubility of amoxicillin B. To stabilise amoxicillin from spontaneous hydrolysis in aqueous solution C. To act synergistically with amoxicillin by inhibiting penicillinases produced by the organism D. To promote increased amoxicillin transport across the outer membrane of the organism E. To promote increased binding affinity of amoxicillin with the bacterial targetarrow_forwardAn experiment was carried out to measure the reaction rate of hydrolysis of acetylcholme (substrate) with serum enzymes (Eadie, 1949). In the experiment, two experiments were conducted, namely experiment 1 without using a prostigmine inhibitor and experiment 2 using a prostigmine inhibitor at 1.5 x 10^-7 mol/l. the data obtained are: a. Is prostigmine competitive or noncompetitive inhibitor? b. determine the value of km and rmax for the two experiments, comparearrow_forward
- a) Determine kcat (in units of sec-1) for a particular enzyme, given the following information: Vo = 144 mmol/min; [S] = 2 mM; Km = 0.5 mM; Enzyme Molecular weight = 40,000 mg/mmole; 8 mg of enzyme used in assay generating this data. b) In general, explain how the total enzyme concentration affects turnover number and Vmax?arrow_forwardSketch on one reaction rate vs. substrate concentration graph & sketch on one Lineweaver-Burk type plot the following:a) A Michaelis-Menten enzyme with a Vmax = 60 1/s and a KM = 125 M.b) An uncompetitive inhibitor of the enzyme described in a).c) An allosteric enzyme with the same Vmax as the enzyme described in a) and follows the sequential modelarrow_forwardA purified protein sample was used in a reaction, resulting in an activity of 696.7 nmol min-1. The reaction volume was 145.0 µL and the final volume before loading the plate was 1,050 µL. The total reaction time was 4.25 min. The amount of protein used in the reaction was 4.270 µg. Calculate the specific activity of the sample (in nmol min-1 µg-1).arrow_forward
- An enzyme that follows Michaelis-Menten kinetics has a KM value of 3.00 µM and a keat value of 181 s1. At an initial enzyme concentration of 0.0100 µM, the initial reaction velocity was found to be 1.07 x 10-0 µM/s. What was the initial concentration of the substrate, S, used in the reaction ? Express your answer in micromolar to three significant figures. > View Available Hint(s) ? [S] !! µM Submitarrow_forwardFrom a kinetics experiment, the Vmax was determined to be 450µM∙min-1. For the kinetic assay, 0.1mL of a 0.05mg/mL solution of enzyme was used, and the enzyme has a molecular weight of 125,000 g/mole. Assume a reaction volume of 700µL. Calculate the kcat (in sec-1) for the enzyme.arrow_forwardWe have mentioned Eadie-Hofstee plots as an alternative to Lineweaver-Burk plots for expression of kinetic data. Sketch what Eadie-Hofstee plots would look like for a series of experiments at different concentrations of (a) a competitive inhibitor (b) a mixed inhibitorarrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





