
Concept explainers
(a)
Interpretation: In the given structure, the relationship between X and
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
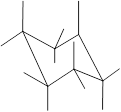
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
In the chair conformation of the cyclohexane, if two groups are pointing in same direction, it is cis conformation and if the two groups are pointing in opposite direction then it is trans conformation.
(b)
Interpretation: In the given structure, the relationship between X and Z group needs to be determined.
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
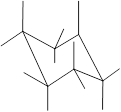
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
In the chair conformation of the cyclohexane, if two groups are pointing in same direction, it is cis conformation and if the two groups are pointing in opposite direction then it is trans conformation.
(c)
Interpretation: In the given structure, the relationship between Y and Z group needs to be determined.
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
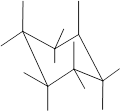
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
In the chair conformation of the cyclohexane, if two groups are pointing in same direction, it is cis conformation and if the two groups are pointing in opposite direction then it is trans conformation.
(d)
Interpretation: In the given structure, the relationship between
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
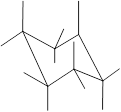
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
In the chair conformation of the cyclohexane, if two groups are pointing in same direction, it is cis conformation and if the two groups are pointing in opposite direction then it is trans conformation.
(e)
Interpretation: In the given structure, the relationship between Z and
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
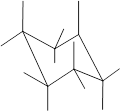
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
In the chair conformation of the cyclohexane, if two groups are pointing in same direction, it is cis conformation and if the two groups are pointing in opposite direction then it is trans conformation.
(f)
Interpretation: In the given structure, the relationship between Z and
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
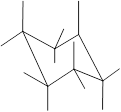
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
In the chair conformation of the cyclohexane, if two groups are pointing in same direction, it is cis conformation and if the two groups are pointing in opposite direction then it is trans conformation.
(g)
Interpretation: In the given structure, the relationship between Y and
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
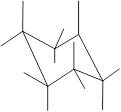
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
In the chair conformation of the cyclohexane, if two groups are pointing in same direction, it is cis conformation and if the two groups are pointing in opposite direction then it is trans conformation.
(h)
Interpretation: In the given structure, the relationship between
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
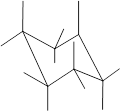
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
In the chair conformation of the cyclohexane, if two groups are pointing in same direction, it is cis conformation and if the two groups are pointing in opposite direction then it is trans conformation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
- 4. The full structural formula of three organic compounds, P, Q and R, are shown below. H H HHH H HHHH H- C-C- H H C C-C = C- H H C C C C-H H H H H H. a. State one similarity between P, Q and R in terms of their molecular formula. b. Name the homologous series that compounds P, Q and R belong to. c. State one similarity between Q and R in terms of chemical bonding. d. Which of these compounds are isomers? Explain your answer.arrow_forwardHow many hydrogens do the following compound have? C9H?NO, has one ring and three double bond a. 13 b. 16 c. 12 d. 11arrow_forwardC. Alkenes 1. Construct a model of ethene (C₂H4) by joining two carbon atoms with a double bond. Toggle between skeletal and full Lewis structures by clicking the C-H tool. Note the trigonal planar geometry of the bonds around each carbon atom, and that the carbon-carbon double bond has restricted rotation. Observe that the six atoms in ethene all lie in the same plane, and the double bond is a fixed geometry within the molecule. 2. Add a CH3- group to each of the doubly-bonded carbons to create a four-carbon alkene with the double bond between carbons 2 and 3. Note that the CH3- groups can be attached in two different ways: Both CH3- groups on the same side of the fixed geometry of the double bond. The two CH3- groups on opposite sides of the double bond. Click the broom to tidy up the structures; convert each to a ball-and-stick model clicking the 2D to 3D tool. Take scr prints of your MolView structures, showing both the Lewis structure and 3D model. ● Below, draw structures for…arrow_forward
- Chemistry Determine the number of electrons that are be capable of delocalizing. :O: :F: O 8 O 12 O 4 O 2 Which set (or sets) of molecules shows two isomers? I. H-ö: II. H H. `C C H. H .O. III. H :Br: IV. H :Br: :Br: H H :Br -с —с- н н-с—с-—Н H H H :Br: O Set III shows a pair of isomers O Sets III and IV both show pairs of isomers O Set I shows a pair of isomers O Set Il shows a pair of isomers O Sets I and IIl both show pairs of isomers Lattice energy relates to which of the following chemical and/or physical properties? O electrolyte strength O acid strength O combustibility O The temperature at which a molecular compound boils エ エarrow_forwardIdentify the functional groups in each molecule. . CH₂ CH,CH,CO2 CH;C=H Darvon (analgesic) CH₂N(CH3)2 b. HO CH₂ - တစ CH₂ COOH penicillin G (an antibiotic) ibuprofen (analgesic) .arrow_forwardV. Give what is asked. 1. When 2 carbon atoms form a double bond, how many pairs of e- will be shared between them? 2. An alkane has 5 carbon atoms. How many hydrogen atoms will it have? 3. An alkene has 3 carbon atoms. How many hydrogen atoms will it have? 4. An alkyne has 4 carbon atoms. How many hydrogen atoms will it have? 5. Show the Lewis structure for the stable molecule CH3.arrow_forward
- Convert each condensed formula to a Lewis structure. a. CH3(CH2)4CH(CH3)2 b. (CH3)3CCH(OH)CH2CH3 c. (CH3)2CHCHO d. (HOCH2)2CH(CH2)3C(CH3)2CH2CH3arrow_forward11. Circle and label all functional groups of the following molecules. a). Nepetalacon, the active ingredient of catnip: HC CH b). Methyl stressonate, isolated from the sweat of students taking organic finals: HC CH, N(CH,)2 QCH3 ÓCH, c). Xenical, a weight loss drug that work by irreversibly blocking stomach and intestinal enzymes involved in fat metabolismarrow_forward***** ******** ******* ******* **** 194 *** Blackboard Learn ← → C **** k tab esc app.aktiv.com X 1 Draw the structure of [CH3CH2CH2CH2NH3]+.. Include all lone pairs and charges as appropriate. 10 F1 My Drive - Google Drive Q Version: 1.163.0+ production Drawing 2 F2 W # 3 X 20 F3 E $ 4 000 000 F4 Courses R % 5 F5 T Problem 1565 of 25 X Atoms, Bonds and Rings MacBook Air 6 Draw or tap a new bond to see suggestions. F6 Aktiv Chemistry Y & Charges and Lone Pairs 7 0 F7 U * 8 DII F8arrow_forward
- 5. Redraw the following structures below in Lewis form, filling in all of the implied carbon atoms and hydrogen atoms. 6. The following are the reagent, intermediate, and product in a reaction you will learn later in Organic Chemistry. a. Identify any formal charges that are missing from the structures. b. Draw in all lone pairs. c. Redraw the structures with all hydrogen atoms. to t OH ₂arrow_forward4. Identify which of the structures below correspond to a. b. C. d. CH,CH,CH,CH(CH3)2 f. (CH3)2(CH2)2CH(CH3)2 (CH3)2CH₂CH₂CH(CH3)2 CH3(CH2)2CH(CH3)2 e. g. h. H H H H—C—C—C—C | H H H с H H H I C-H CIHarrow_forwardHere is the chemical structure of 2-bromobutane: H H. H C-C-H H :Br: H H Decide whether each molecule in the table below is another molecule of 2-bromobutane, a molecule of an isomer of 2-bromobutane, or a molecule of an entirely different compound. molecule relationship to 2-bromobutane CH, (Choose one) H,c=ċ=CH,-Br Explanation Check O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center I Accessibility P Type here to search 76°F DELL F1 F2 F3 F4 F5 FB F9 F10 F11 F12 PrtScr PAA @ %23 2$ 1 8. 9 Q W R Y. A F G H. K V N4 Alt Ctri Alt Σ B. C1arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

