
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 7, Problem 16CTQ
Interpretation Introduction
Interpretation: In the given structures, each black ball needs to be labeled as up or down.
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
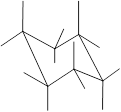
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
The axial and equatorial groups are represented in the chair conformation as A and E:
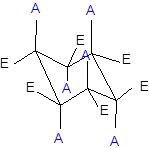
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
7. Help
11. Help
8. Help
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 7 - Prob. 1CTQCh. 7 - Prob. 2CTQCh. 7 - Prob. 3CTQCh. 7 - Draw wedge and dash skeletal representations of...Ch. 7 - Label each ring in Figure 7.2 cis or trans.Ch. 7 - Prob. 6CTQCh. 7 - Prob. 7CTQCh. 7 - Prob. 8CTQCh. 7 - a model of cyclohexane in a chair conformation,...Ch. 7 - Prob. 10CTQ
Ch. 7 - Prob. 11CTQCh. 7 - Fill in the blanks: cis-1,3-Dimethylcyclohexane...Ch. 7 - Prob. 13CTQCh. 7 - Prob. 14CTQCh. 7 - Prob. 15CTQCh. 7 - Prob. 16CTQCh. 7 - Prob. 17CTQCh. 7 - Prob. 18CTQCh. 7 - Draw chair representations of...Ch. 7 - Which stereoisomer in the previous question is...Ch. 7 - Prob. 21CTQCh. 7 - Prob. 1ECh. 7 - Label each of the following as cis, trans or...Ch. 7 - Which pair has more in common with one another?Ch. 7 - Prob. 4ECh. 7 - Prob. 5ECh. 7 - Prob. 6ECh. 7 - Fill in the table by drawing a representation of a...Ch. 7 - Prob. 9ECh. 7 - True or False: If you perform a chair flip on...Ch. 7 - Prob. 11ECh. 7 - Prob. 12ECh. 7 - Prob. 13ECh. 7 - Prob. 14ECh. 7 - Prob. 15ECh. 7 - Draw trans-1-tert-butyl-3-methylcyclohexane in its...Ch. 7 - Build a model of methylcyclohexane, and use the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following are descriptions of possible starting material for this reaction? H ? trace acid an ester a ketone an imine an aldehyde a carboxylic acid an enamine a primary amine a secondary amine a tertiary aminearrow_forwardNonearrow_forwardWhat are the reagents needed for this and the third structure I only got the top right structure rightarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY