
Concept explainers
(a)
Interpretation: The one which changes during a chair flip in case of cyclohexane and the one which remains the same needs to be explained.
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
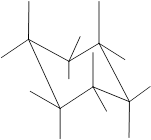
The groups showing in straight upward and downward directions are axial, and the groups bend slightly right or left are equatorial.
The axial and equatorial groups are represented in the chair conformation as A and E:
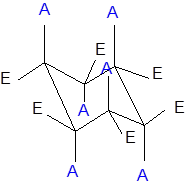
(b)
Interpretation: The change in the group that is up and in axial position in the cyclohexane needs to be explained after the chair flip.
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
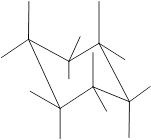
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
The axial and equatorial groups are represented in the chair conformation as A and E:
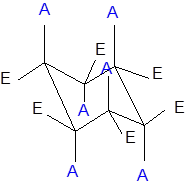
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
- I. Each -OH group of an alcohol can stabilize four to five carbon atoms.II. The boiling point of alcohol is lower than the boiling point of ethers of comparable mass.A. FIRST statement is INCORRECT, SECOND is CORRECTB. BOTH statements are CORRECTC. FIRST statement is CORRECT, SECOND is INCORRECTD. BOTH statements are INCORRECTarrow_forwardHomolysis of the indicated C–H bond in propene forms a resonancestabilized radical. a.Draw the two possible resonance structures for this radical. b.Use half-headed curved arrows to illustrate how one resonance structure can be converted to the other. c. Draw a structure for the resonance hybrid.arrow_forward. Consider the hash/wedge structure below. a. Draw the Newman projection of the bond indicated, keeping the conformation the same. НО b. Draw the Newman projection for the most stable conformation CI c. In the Newman projection for part b, label all gauche interactions.arrow_forward
- 1. Draw the most and least stable Newman projections for the following molecules (focus on C2 and C3). a. 2-chloro-2-fluoropentane b. 2,2-dimethylbutane c. 2-chloro-2-methylpentane d. 1,2-dibromoethanearrow_forwardDraw a model of ethane. Imagine rotating the C-C single bond. a. Is there a change in the relative positions of the different atoms as the C-C bond is rotated? Yes or No? b. Does the ethane molecule have more than one conformation? Yes or No?arrow_forwardCircle the correct “Class” name for each pair.1. Aromatic or benzene ring2. Hydroxyl or alcohol3. Single bond or Alkane4. Thiol or sulfhydryl5. Carbonyl or Aldehyde Circle the correct “Functional group” name for each pair6. Triple bond or Alkyne7. Alcohol or hydroxyl8. Amine or Amino9. Carbonyl or Ketone10. Ether or Alkoxyarrow_forward
- OH a. Draw the cis and trans isomer of the molecule above. b. Draw the chair form of the trans isomer of the molecule. C. Draw the flipped chair form of the trans isomer of the molecule. d. What is the more stable chair form?arrow_forward\Br A. Redraw the bond-line structure of the molecule above on a separate sheet of paper. B. Draw a chair conformation for the molecule. C. Draw the new chair conformation after ring inversion (ring flip). D. Circle the most stable (lowest energy) chair conformation.arrow_forwardChemistry Questionarrow_forward
- a. Draw the Newman projection for each molecule shown here, looking down the c-c bond indicated by the arrow. b. Which configuration do you think is more stable? Explain.arrow_forwardThe majority of cis-3-isopropyl-1-methylcyclohexane molecules assume a conformation wherein a. both isopropyl and methyl groups are equatorial. b. the methyl group is axial and the isopropyl group is equatorial. c. the isopropyl group is axial and the methyl group is equatorial. d. both isopropyl and methyl groups are axial.arrow_forwardHow are the molecules below related to each other? (Hint: convert each to a partially condensed structual formula). Н. H H CH3 H₂C CH3 CH3 and H H H H₂c-CH₂ CH3 H CH3 Select one: OA. They are constitutional isomers. OB. They are identical compounds. O C. They are geometrical isomers. O D. They are stereoisomers. O E. They are different compounds, not isomers..arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
