
Concept explainers
(a)
Interpretation: The molecule with the lowest potential energy needs to be drawn for the following condition:
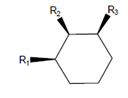
Here,
Concept Introduction: The molecule with lowest potential energy is most stable. In the chair conformation of the cyclohexane, the stable conformation is when large groups are present in the equatorial positions. The equatorial positions in the chair conformation points away from the ring thus, there is less steric hinderance.
(b)
Interpretation: The molecule with the lowest potential energy needs to be drawn for the following condition:
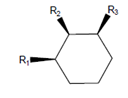
Here,
Concept Introduction: The molecule with lowest potential energy is most stable. In the chair conformation of the cyclohexane, the stable conformation is when large groups are present in the equatorial positions. The equatorial positions in the chair conformation points away from the ring thus, there is less steric hinderance.
(c)
Interpretation: The molecule with the lowest potential energy needs to be drawn for the following condition:
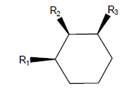
Here,
Concept Introduction: The molecule with lowest potential energy is most stable. In the chair conformation of the cyclohexane, the stable conformation is when large groups are present in the equatorial positions. The equatorial positions in the chair conformation points away from the ring thus, there is less steric hinderance.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
- .....Draw the Newman pr0jecti0ns 0f the three p0ssible staggered c0nf0rmati0ns 0f2,3-dimethylbutane, viewed thr0ugh the C2—C3 b0nd. What are the relativeenergies of each c0nf0rmati0n?arrow_forwardFor the following two conformations of 2,3-dimethylbutane, determine (choose 1 option below) the total energy cost associated with all torsional strain and steric strain. 1. a) (6 kJ/mol x 2) + (11 kJ/mol x3) = 45 kJ/mol b) 3.8 kJ/mol x 4 = 15.2 kJ/mol c) 3.8 kJ/mol x 3 = 11.4 kJ/mol d) 11kJ/mol x 4 = 44 kJ/mol 2. a) 6 kJ/mol + 11 KJ/mol= 17 kJ/mol b) 3.8 kJ/mol x 3 = 11.4 kJ/mol c) (6kJ/mol x 2 ) 3.8 kJ/mol = 15.8 kJ/mol d) (6kJ/mol x 2 ) + 11kJ/mol = 23 kJ/molarrow_forwardMultiple Choice O O O 11 ||| IVarrow_forward
- Chemistry blem 5-6 Star (") each asymmetric carbon atom in the following examples, and determine whether it has the (R) or (S) configuration. CH, HO C a. H CH,CH, H Br C b. CH₂CH, CHO CCH=CH₂ (CH,O),CH CH(CH₂)₂ Please provide explanation, especially on F, I'm not sure how that one has two chiral centers. Thank you! C. d. e. f. 9. h. H,C H H H CI H CI H H CH, H H D Cl H CH, C CH₂arrow_forwardnces IMalings REVIEW VIew E. 三 2 T Aa - AaBbCc[ AaBbCc[ AaBbCc[ AaBbC ay - A 三,這, T Normal 1 Body Text 1 List Para... T No Sp Paragraph Styles Q2. Draw all possible Newman projections for the below structure by sighting along the indicated bond. Arrange the relative stability of all conformations by assigning a number (1 for the least stable and highest number for the most stable) C3-C-4arrow_forwardto Medical Chemistry II. (BMC1CHEM02) oard / My courses / Introduction to Medical Chemistry II. (BMCICHEM02) / 29/03 2021 Chemistry Electronic te Electronic test in Chemistry n3 Cyclobutane is more stable molecule than cyclopropane, because.... Select one: ut of it has less angle strain. cyclobutane has a cis-trans stereoisomers. cyclobutane is a larger ring. it has more torsional strain. cyclopropane is planar. Next pagearrow_forward
- 2. For the following structure: (1S, 2R, 4R)-2-chloro-1-isopropyl-4-methylcyclohexane a. draw both chair conformations for the molecule b. identify whether the more stable stereoisomer.arrow_forwardA. Circle the following molecules that have the S configuration. CH,CH3 CH,CH3 H. H3C-- H. CH=CH2 CH(CH3)2 CH3 CH3CH, CH2 HO, 3 1arrow_forwardCircle the following molecules that have the S configuration. H CH,CH; CH,CH; CH3 ОН CH=CH2 CH3 H;C- H CH;CH, H CH(CH3)2 1 2 3 4.arrow_forward
- Consider rotation about the indicated bond in the molecule below. Which Newman Projection formula best represents the most stable conformer of the molecule with respect to rotation about the indicated bond? [As noted below: there is no steric strain between -H and -Et that have a gauche, staggered relationship] H CH3 Br-C-C-CH₂CH3 H. H Br CH₂CH₂ 1 CH3 H CH3CH₂ H Br H₂C || H H₂C H Br H ||| Select one: OA. I O B. II O C. III O D. All conformers have identical energies. CH₂CH3 H NOTE CH3CH₂ H no steric strainarrow_forward6. Newman projections to Lewis structures From the following Newman projections, draw the corresponding Lewis structure in the same conformation. A) B) H. H CH3 H₂C N H OH CI Br CH3 H OH CH3arrow_forward4. Which Newman projection corresponds to point A on the graph of potential energy vs. rotation about the C₂-C3 bond? Potential Energy- (A) (C) 0 H CH3 Q. 'Н H H H₂ H 60 CH₁ H -CH3 H A CH3 120 180 Degrees of Rotation (B) (D) n 300 240 HH H H 360 CH3 CH3 CH3 HDCH3 H&H Harrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





