
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 2E
Label each of the following as cis, trans or neither. Below each structure that is cis draw a transconfigurational stereoisomer or vice versa.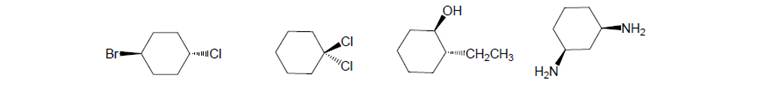
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Classification of boranes.
What is the pH of a solution made by adding 10-2 M sodium benzoate (C6H5COONa) to pure
water, taking into account nonideal solute behavior? Benzoate is the conjugate base of
benzoic acid (Ka = 6.25×10-5), a common preservative added to food and beverages.
Show work. don't give Ai generated solution
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 7 - Prob. 1CTQCh. 7 - Prob. 2CTQCh. 7 - Prob. 3CTQCh. 7 - Draw wedge and dash skeletal representations of...Ch. 7 - Label each ring in Figure 7.2 cis or trans.Ch. 7 - Prob. 6CTQCh. 7 - Prob. 7CTQCh. 7 - Prob. 8CTQCh. 7 - a model of cyclohexane in a chair conformation,...Ch. 7 - Prob. 10CTQ
Ch. 7 - Prob. 11CTQCh. 7 - Fill in the blanks: cis-1,3-Dimethylcyclohexane...Ch. 7 - Prob. 13CTQCh. 7 - Prob. 14CTQCh. 7 - Prob. 15CTQCh. 7 - Prob. 16CTQCh. 7 - Prob. 17CTQCh. 7 - Prob. 18CTQCh. 7 - Draw chair representations of...Ch. 7 - Which stereoisomer in the previous question is...Ch. 7 - Prob. 21CTQCh. 7 - Prob. 1ECh. 7 - Label each of the following as cis, trans or...Ch. 7 - Which pair has more in common with one another?Ch. 7 - Prob. 4ECh. 7 - Prob. 5ECh. 7 - Prob. 6ECh. 7 - Fill in the table by drawing a representation of a...Ch. 7 - Prob. 9ECh. 7 - True or False: If you perform a chair flip on...Ch. 7 - Prob. 11ECh. 7 - Prob. 12ECh. 7 - Prob. 13ECh. 7 - Prob. 14ECh. 7 - Prob. 15ECh. 7 - Draw trans-1-tert-butyl-3-methylcyclohexane in its...Ch. 7 - Build a model of methylcyclohexane, and use the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly explain the existence of Nb-Nb bond in the alpha-NbI4 compound.arrow_forwardIn the case of isopilianions, briefly state:- why polymeric species with a defined MW are formed.- why the extent of polymerization is different depending on the metal.- why these polyhedra with such special structures are formed.arrow_forwardA carboxylic acid reacts with water to form a carboxylate ion and H,O+. Complete the reaction. reaction: (CH),CHCH2COOH + H2O (CH), CHCH, COO¯ + H₂O+ Write the IUPAC name of the carboxylate ion formed in the reaction. IUPAC name: BIU X2 SPECIAL GREEK ALPHABET ~ Iarrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardA solution contains 10-3 M (NH4)2CO3 plus 10-3 M CaCO3. (NH4+: pKa 9.26) a) Follow the four steps and list the species and equations that would have to be solved to determine the equilibrium solution composition. (15 pts) b) Prepare a log C-pH diagram for the solution. Use a full sheet of graph paper, and show the ranges 1≤ pH < 13 and -10≤ log C≤ -1. (10 pts) c) Use the graphical approach for the solution pH. What is the concentration of all species? (15 pts)arrow_forwardKeggin structure.arrow_forward
- Given: N2(g) + 3H2(g)2NH3(g) AG° = 53.8 kJ at 700K. Calculate AG for the above reaction at 700K if the reaction mixture consists of 20.0 atm of N2(g), 30.0 atm of H2(g), and 0.500 atm of NH3(g). A) -26.9 kJ B) 31.1 kJ C) -15.6 kJ D) 26.9 kJ E) -25.5 kJarrow_forwardExplain the structure of the phosphomolybdate anion [PMo12O40]3-.arrow_forwardg. NaI, H3PO4 h. 1. BH3/THF 2. H₂O2, OH i. HC1 j. Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License