
Concept explainers
Interpretation: The structure of cis-1, 3-dimethylcyclohexane needs to be drawn in the chair conformation and the same molecule needs to be drawn after the chair flip.
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
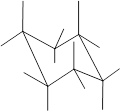
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
During the flipping, no bond is break. The numbering in the chair form is represented as follows:
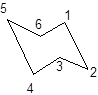
During ring flipping, mirror image of the chair conformation is formed.
It is represented as follows:
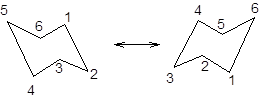
Now, the ring flipping with groups attached can be represented as follows;
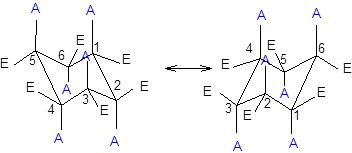
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
- Draw cis and trans chair conformations for 1,4- dimethylcyclohexane. Comment on what happens when a "chair - chair flip" occurs and draw both chair forms and circle the most favorable conformation. Also, comment on the types of strain present.arrow_forwardDraw the Newman projection so that it corresponds to the molecule and conformation shown when viewed down the red bond in the direction of the red arrow. Your projection should be oriented as shown by the arrow marked up. So the CH2CH2CH3 group on the front carbon should be below the H3C and H groups, no matter which template you use. H H₁C up 三arrow_forwardc) Draw the most stable chair conformation for each of the following cyclohexanes. Now, "flip" the ring and redraw the molecule in the higher energy form. i) chlorocyclohexane ii) cis-1-chloro-2-methylcyclohexane iii) trans-1-methyl-3-propylcyclohexanearrow_forward
- Draw the Newman projection so that it corresponds to the molecule and conformation shown when viewed down the red bond in the direction of the arrow. H I H CH3 X 5 Carrow_forward4. Draw a chair conformation of the following molecules. Ring flip your original chair, and circle the more stable conformation. cis-1,2-dimethylcyclohexane trans-1-tert-butyl-3-ethylcyclohexane cis-1,4-diisopropylcyclohexane cis-1-butyl-3-methylcyclohexanearrow_forwardDraw a chair conformation of this molecule with (a) all CH3 groups in axial positions and (b) all CH3 groups in equatorial positions.arrow_forward
- Font Paragraph Using a Newman projection, draw the most stable conformation of CH2-CH2. | ОН ОНarrow_forwardCreate a conformational analysis using the molecule in the picture. Follow the instruction in the picture when you transform the molecule into its Newman projection. Which conformer is the most stable? Explain.arrow_forwardDraw all the conformers of the molecule on the right as Newman projections sighted along the C3– C4 bond. Label the interactions present in each conformer. Rank the conformers from lowest to highest energy (some may be equal).arrow_forward
- The sawhorse projection shown below depicts this molecule in a specific conformation. Draw a Newman projection of this molecule in the same conformation. H H_H- CH3 CH3 CH3arrow_forwarda) draw the newman projectjon between C3-C4 bond for all of the structures over 360 degrees of rotation. start with highest energy of conformation b) starting at 0 degrees draw a qualitative torsional energy plot as a function of degree of rotationarrow_forwardDraw the “chair twist" of the following molecules. Label the more stable conformation. Br •CH3 OH Draw planar models of the above molecules. Draw both chair conformation of the following molecule, and identify the most stable form. Me Me ....Et Mearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
