
(a)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted from the given multistep reactions:
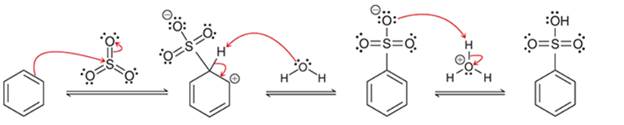
Concept introduction:
A
(b)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions.
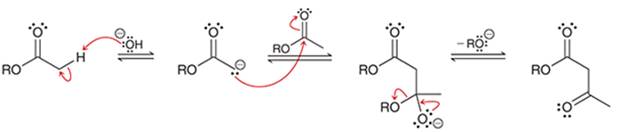
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
(c)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions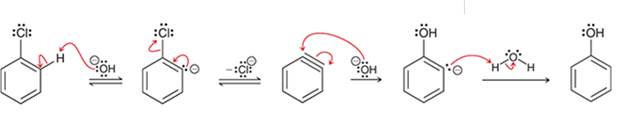
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
(d)
Interpretation: The sequence of arrow pushing patterns of the curved arrows is to be interpreted for the given multistep reactions.
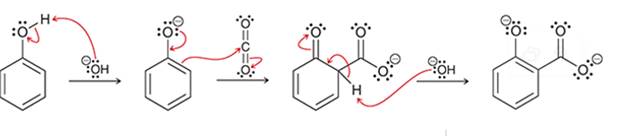
Concept introduction:
A chemical reaction can be completed in more than one step. Such reactions are called multistep reactions. It occurs with the formation of intermediates. The formation of intermediate can be shown with the help of curved arrows. Intermediates like carbocation and arenium cations are common intermediates.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- (a) Select all of the correct statements about reaction rates from the choices below. The lower the rate of a reaction the longer it takes to reach completion.Reactions involving very unstable combinations of chemicals have large rate constants.Concentrations of homogeneous catalysts have no effect on reaction rates.Reaction rate constants are independent of temperature.The slowest step in a reaction is called the rate-determining step.A balanced chemical reaction is necessary to relate the rate of reaction to the concentration of a reactant.Slow reactions can be speeded up by raising the temperature.arrow_forward1 Rate constants for the first-order decomposition of acetonedicarboxylic acid CO(CH2COOH)2(aq) → CO(CH3)2(aq) + 2 CO2(g) acetonedicarboxylic acidacetone are k = 4.75 ×10–4 s–1 at 293 K and k = 1.63 ×10–3 at 303 K. What is the activation energy, Ea, for this reaction? Select one: a. 71KJ/mol b. 81KJ/mol c. 51KJ/mol d. 91kJ/molarrow_forwardThe data below show the concentration of N2O5 versus time for the following reaction: N2O5 (g) → NO3 (g) + NO2(g) Time (s) [N2O5] (M) 1.000 25 0.822 50 0.677 75 0.557 100 0.458 125 0.377 150 0.310 175 0.255 200 0.210arrow_forward
- Cleavage of C2H6 to produce two CH3· radicals is a gas-phase reaction that occurs at 700 °C. This reaction is first order, with k = 5.46 × 10−4 s−1. Write down the chemical equation for the cleavage reaction and the rate equations.arrow_forwardно HO HO HO 0 + ½ O, → + H,0 H H но он ascorbic acid dehydroascorbic acid Vitamin C is oxidized slowly to dehydroascorbic acid by the oxygen in air. It is catalyzed by ions such as Cu*2 and Fe*3. The reaction can be followed by measuring the ultraviolet absorbance at 243 nm. Time (hours) Absorbance (A) 1/A In A - In A 0.75 1.3 -0.29 0.29 1 0,38 2.6 -0.97 0.97 2 0.19 5.3 - 1.7 1.7 3 0.095 11 - 2.4 2.4 28. A new compound is synthesized and found to be a monoprotic acid with a molar mass of 248 g/mole. When 0.0050 moles of this acid are dissolved in 0.500L of water, the pH is measured as 2.94. What is the pKa of this acid? (A) (B) (C) (D) 2.33 3.89 5.78 7.78 29. What is the hybridization of carbon 1 (far left) and carbon 2 (middle) in this hydrocarbon: CH3CH=CH2? (A) sp³, sp (B) sp?, sp? (C) sp³, sp? (D) sp, sp?arrow_forwardWhat is the order of reaction in regards to Cl2?arrow_forward
- The formation of tert-butanol is described by the following chemical equation: (CH3)₂CBr (aq) + OH(aq) → Br¨¯ (aq) + (CH3)₂COH(aq) Suppose a two-step mechanism is proposed for this reaction, beginning with this elementary reaction: (CH³)¸CBr (aq) → (CH3)¸₂C² (aq) +Br¯(aq) Suppose also that the second step of the mechanism should be bimolecular. Suggest a reasonable second step. That is, write the balanced chemical equation of a bimolecular elementary reaction that would complete the proposed mechanism. 0 ロ→ロ Śarrow_forward(4) In the upper atmosphere, oxygen exists in forms other than O2(g). For example, it exists as ozone, Os(g), and as single oxygen atoms, O(g). Ozone and atomic oxygen combines to form two molecules of oxygen. For this reaction, the enthalpy change is -392 kJ and the activation energy is 19kJ. Draw and label a potential energy diagram. Include a value for Ea (rev). Propose a strusazcture for the activated complex.arrow_forwardThe reactions drawn show possible arrow pushing mechanisms. Considering what you know about arrow pushing mechanisms, identify which show valid arrow pushing. Select the valid mechanisms. X-Y X=Y X=Y + Z X-Y-Z → X-Y X + Y X-Y X-Y X-Y + Z' X-Y-Zarrow_forward
- A suggested mechanism for the decomposition of peroxide (H2O2) is H2O2(aq) ->>> 2OH(aq) H2O2(aq) + OH(aq) H2O()+ HO2(g) HO2(g) + OH(aq)) → H2O(l) + O2 (g) ->> Based on the mechanism which statement is true? (1) The rate law is rate = k[H202] (2) the molecularity of the slow reaction is 4 (4) OH2 is an intermediate (3) OH is a catalyst A Moving to the next question prevents changes to this answer. MacBook Pro fast slow fastarrow_forwardFor the gas phase isomerization of cis-1,2-diphenylethene, cis-C6H5 CH=CHC6H5 → trans-C6H5 CH=CHC6H5 the rate constant at 569 K is 2.11 × 10-4 s¯¹ and the rate constant at 608 K is 2.39 × 10 -3 S The activation energy for the gas phase isomerization of cis-1,2-diphenylethene is | s-¹. S kJ.arrow_forward(a) Select all of the correct statements about reaction rates from the choices below. The lower the rate of a reaction the longer it takes to reach completion. The fastest step in a reaction is called the rate-determining step. As a reaction progresses its rate goes up. The rate of a reaction is independent of temperature. As a reaction progresses its rate goes down. Reactions involving very unstable combinations of chemicals have small rate constants. O Reaction rates decrease with increasing temperature. xarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

