
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6, Problem 55IP
Interpretation Introduction
Interpretation: The missing lone pairs and curved arrows is to be interpreted for each step of the given mechanism.
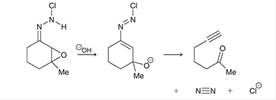
Concept introduction: In a
The curved arrows help to determine the movement of electrons, bonds, and reagents. It also shows the attack of electrophile or nucleophile along with loss of leaving group.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the Newman projection so that it corresponds to the molecule and conformation shown when viewed down the red bond in the direction of the red arrow. Your projection should be oriented as shown by the arrow marked up. So the CH2SH group on the front carbon should be above the H and H3C groups, no matter which template you use
Draw the Newman projection so that it corresponds to the molecule and conformation shown when viewed down the red bond in the direction of the arrow.
н с H
H CH,
4. Draw a Newman projection of the following compound that centers along the marked
bond.
This one!
CH3
CH3
+ +
H
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Ch. 6.1 - Prob. 1LTSCh. 6.1 - Prob. 1PTSCh. 6.1 - Prob. 2ATSCh. 6.2 - Prob. 3CCCh. 6.3 - Prob. 4CCCh. 6.3 - Prob. 5CCCh. 6.4 - Prob. 6CCCh. 6.6 - Prob. 7CCCh. 6.7 - Prob. 2LTSCh. 6.7 - Prob. 8PTS
Ch. 6.7 - Prob. 9PTSCh. 6.7 - Prob. 10ATSCh. 6.8 - Prob. 3LTSCh. 6.8 - Prob. 11PTSCh. 6.8 - Prob. 12ATSCh. 6.9 - Prob. 4LTSCh. 6.9 - Prob. 13PTSCh. 6.9 - Prob. 14ATSCh. 6.10 - Prob. 5LTSCh. 6.10 - Prob. 15PTSCh. 6.10 - Prob. 16ATSCh. 6.11 - Prob. 6LTSCh. 6.11 - Prob. 17PTSCh. 6.11 - Prob. 18ATSCh. 6 - Prob. 19PPCh. 6 - Prob. 20PPCh. 6 - Prob. 21PPCh. 6 - Prob. 22PPCh. 6 - Prob. 24PPCh. 6 - Prob. 25PPCh. 6 - Prob. 26PPCh. 6 - Prob. 27PPCh. 6 - Prob. 28PPCh. 6 - Prob. 29PPCh. 6 - Prob. 30PPCh. 6 - Prob. 31PPCh. 6 - Prob. 32PPCh. 6 - Prob. 33PPCh. 6 - Prob. 34PPCh. 6 - Prob. 35PPCh. 6 - Prob. 36PPCh. 6 - Prob. 37PPCh. 6 - Prob. 38PPCh. 6 - Prob. 39PPCh. 6 - Prob. 40PPCh. 6 - Prob. 41PPCh. 6 - Prob. 43ASPCh. 6 - Prob. 44ASPCh. 6 - Prob. 45ASPCh. 6 - Prob. 46ASPCh. 6 - Prob. 47ASPCh. 6 - Prob. 48ASPCh. 6 - Prob. 49ASPCh. 6 - Prob. 50IPCh. 6 - Prob. 51IPCh. 6 - Prob. 52IPCh. 6 - Prob. 53IPCh. 6 - Prob. 54IPCh. 6 - Prob. 55IPCh. 6 - Prob. 56IPCh. 6 - Prob. 57IPCh. 6 - Prob. 58IPCh. 6 - Prob. 59IPCh. 6 - Prob. 60IPCh. 6 - Prob. 61IPCh. 6 - Prob. 62CPCh. 6 - Prob. 64CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4) Draw the three staggered Newman projections for the following compound and circle the most stable.arrow_forwardDraw the Newman projection so that it corresponds to the molecule and conformation shown when viewed down the red bond in the direction of the red arrow. Your projection should be oriented as shown by the arrow marked up. So the CH2CH2CH3 group on the front carbon should be below the H3C and H groups, no matter which template you use. H H₁C up 三arrow_forwardDraw the curved arrows that accomplish each of the following transformations. H O-H H3C H3C ∙H CH3 (+ H3C CH3 CH3 Edit Drawing Harrow_forward
- . Use the curly arrow formalism to illustrate how the transformations shown below might occur. CH₂ (+) A CH₂arrow_forwardDraw the curved arrows that accomplish each of the following transformations. H by H CH3 Lave CH3 + CH3 H3C H Edit Drawing O-H H3C H3C +H CH3 CH3arrow_forward5. Draw the Anti, Gauche, and Eclipsed Newman projections of hexane looking down the C3-C4 bond. Circle with one with the lowest energy.arrow_forward
- 2) Provi c) In the below compounds, circle the most stable and cross out the least stable - CH3arrow_forwardDraw each of the following. (a) Draw a Newman Projection of the following molecule, looking down the C3-C4 bond. Draw C3 in the front. CI Br H (b) Draw a Newman Projection of the most stable and the least stable conformation of the following molecule. Label each H H. CI H CI Harrow_forwardDraw the skeletal structure of a neutral acyclic molecule with one tetrahedral stereocenter. You may use only 8 carbon atoms and 1 halogen atom, plus as many hydrogen atoms as you like. Click and drag to start drawing a structure. S Shm Carrow_forward
- Then draw the most stable and least stable Newman projection conformation from the C4-C5 bond in the molecule abovearrow_forwardConsider the sugar below, one of several sugars in the antibiotic streptomycin. Draw in all of the hydrogens and label them as axial or equatorial. Reverse, reverse!Draw the molecule with traditional wedge/dash notation. Draw a Newman projection looking down the indicated bond. Practice self- care by abbreviating the ring when doing so.arrow_forwarda) Sighting down the C3-C4 bond, draw the gauche (60 degrees) and anti (180 degrees) Newman projections of 2,4-dimethylhexane. b) Circle the conformation that you drew that is lower energy.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY