
Concept explainers
(a)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
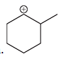
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(b)
Interpretation: A carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
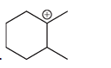
Concept introduction: A carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(c)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
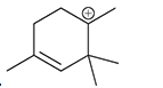
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(d)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
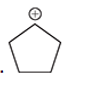
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given based on the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(e)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.

Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on the primary, secondary, and tertiary carbon atoms of the intermediate.
(f)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
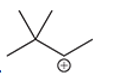
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on primary, secondary, and tertiary carbon atoms of the intermediate.
(g)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
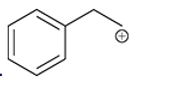
Concept introduction: A carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on primary, secondary, and tertiary carbon atoms of the intermediate.
(h)
Interpretation: Carbocation can rearrange to form a stable carbocation. The carbocation rearrangement with a curve arrow is to be interpreted for the given carbocation.
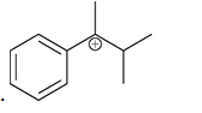
Concept introduction: Carbocation is an intermediate that carries a positive charge on the carbon atom. It tends to rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary >Secondary >Primary
The notation to carbocation is given on the basis of the positive charge on primary, secondary and tertiary carbon atom of the intermediate.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Draw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. _NH2 NH2 Br2 Br • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one.arrow_forwardCan this carbocation rearrange to a more stable one?arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. You do not have to consider stereochemistry. Include all valence lone pairs in your answer.arrow_forward
- 9. For the following carbocations: 1) determine if they rearrange, and 2) if they rearrange, draw the expected product and the curved arrows to complete the rearrangement.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the product of this carbocation rearrangement. Include all lone pairs.arrow_forwardCO2 Follow the curved arrows and draw the product of this reaction. You do not have to consider stereochemistry.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Follow the arrows and draw the intermediate and product in this reaction. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts. Draw Intermediate CH₂OH₂* protonation H₂C CH3OH₂* protonation CH3OH deprotonation loss of H₂O elimination Draw Intermediate CH₂OH nucleophilic addition || Draw Intermediate CH3OH deprotonation Draw Productarrow_forwardFor each set of conditions, circle the favored reaction(s) and draw the major product(s). Assume that these reactions are irreversible. If both products are expected to form in comparable yields, circle both reactions and draw both products.arrow_forwardAlkene bromination. The two butane isomers react with Br2. Draw the key reaction inter- mediates and the product(s); use arrow pushing to explain the stereochemical outcomes of the reactions Final Products Intermediate Br2 Intermediate Final Products Br2arrow_forward
- The benzyl carbocation is a resonance-stabilized carbocation similar to the allyl carbocation. One resonance structure of the benzyl carbocation is shown here. There are three other resonance structures in which the positive charge is distributed over three carbons in the benzene ring. What are the other resonance structures?arrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown. Please circle the one I need to draw in the space below.arrow_forwardDraw the product(s) of the reaction. Include all lone pairs.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
