
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Question
Chapter 6.9, Problem 14ATS
Interpretation Introduction
Interpretation: The arrow pushing pattern for the given mechanism is to be interpreted for the conversion of compound 1 to 2.
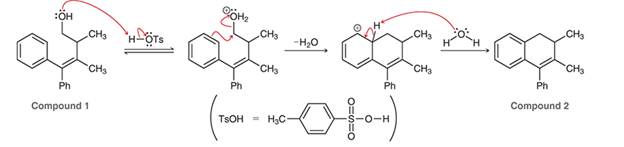
Concept introduction: In a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Under acid-catalyzed conditions, epoxides can be opened by a variety of nucleophiles other than water, such as alcohols. In such a
case, the nucleophile will generally attack at the more substituted position. Using this information, predict the products for each
of the following reactions (a-b):
?
1) RCO-H
2) [H₂SOJ
Modify the given structure to draw the major product(s). Use the single bond tool to interconvert between double and single
bonds. If a racemic mixture of enantiomers is expected, draw both enantiomers. Note: you can select a structure and use Copy and
Paste to save drawing time.
CH₂
Edit Drawing
A
The compound below was exposed to a palladium catalyst to produce a macrocycle (large ring). The macrocycle subsequently
underwent a rearrangement to produce a fused hexacyclic (six-ring) structure. The ring system of the final product is one that is found
in a number of polycyclic natural products. Draw the initial product of the palladium-catalyzed reaction, the final product, and a
mechanism consistent with the rearrangement step.
Include stereochemistry in your answer.
SnBu,
TIO
Integrated Problem 23.67a
* Your answer is incorrect.
Draw the initial product of the palladium-catalyzed reaction:
Optically pure Compound 1 undergoes a reaction at room temperature with sodium methoxide (NaOCH3) in methanol to form a single isomer of Compound 2 as shown below:
1. What are the stereochemical designations (R or S) of Compound 1 and Compound 2?
2. On the basis of the structure of Compound 2 and the information on the reaction conditions, suggest which type of mechanism Compound 1 undergoes .
3. The rate of the above reaction is determined experimentally to follow second-order kinetics. Give a fully labelled sketch of a reaction coordinate diagram for the reaction.
4. draw a mechanism on a piece of paper (using curly arrows) to show the formation of Compound 2 from Compound 1 including any activated complex.
5. If the sodium methoxide is left out of the reaction mixture, Compound 2 is formed in roughly equal amounts with another compound (Compound 3). Suggest a structure for Compound 3. With reference to the mechanism of the reaction and the structure of Compound 1, explain how these…
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Ch. 6.1 - Prob. 1LTSCh. 6.1 - Prob. 1PTSCh. 6.1 - Prob. 2ATSCh. 6.2 - Prob. 3CCCh. 6.3 - Prob. 4CCCh. 6.3 - Prob. 5CCCh. 6.4 - Prob. 6CCCh. 6.6 - Prob. 7CCCh. 6.7 - Prob. 2LTSCh. 6.7 - Prob. 8PTS
Ch. 6.7 - Prob. 9PTSCh. 6.7 - Prob. 10ATSCh. 6.8 - Prob. 3LTSCh. 6.8 - Prob. 11PTSCh. 6.8 - Prob. 12ATSCh. 6.9 - Prob. 4LTSCh. 6.9 - Prob. 13PTSCh. 6.9 - Prob. 14ATSCh. 6.10 - Prob. 5LTSCh. 6.10 - Prob. 15PTSCh. 6.10 - Prob. 16ATSCh. 6.11 - Prob. 6LTSCh. 6.11 - Prob. 17PTSCh. 6.11 - Prob. 18ATSCh. 6 - Prob. 19PPCh. 6 - Prob. 20PPCh. 6 - Prob. 21PPCh. 6 - Prob. 22PPCh. 6 - Prob. 24PPCh. 6 - Prob. 25PPCh. 6 - Prob. 26PPCh. 6 - Prob. 27PPCh. 6 - Prob. 28PPCh. 6 - Prob. 29PPCh. 6 - Prob. 30PPCh. 6 - Prob. 31PPCh. 6 - Prob. 32PPCh. 6 - Prob. 33PPCh. 6 - Prob. 34PPCh. 6 - Prob. 35PPCh. 6 - Prob. 36PPCh. 6 - Prob. 37PPCh. 6 - Prob. 38PPCh. 6 - Prob. 39PPCh. 6 - Prob. 40PPCh. 6 - Prob. 41PPCh. 6 - Prob. 43ASPCh. 6 - Prob. 44ASPCh. 6 - Prob. 45ASPCh. 6 - Prob. 46ASPCh. 6 - Prob. 47ASPCh. 6 - Prob. 48ASPCh. 6 - Prob. 49ASPCh. 6 - Prob. 50IPCh. 6 - Prob. 51IPCh. 6 - Prob. 52IPCh. 6 - Prob. 53IPCh. 6 - Prob. 54IPCh. 6 - Prob. 55IPCh. 6 - Prob. 56IPCh. 6 - Prob. 57IPCh. 6 - Prob. 58IPCh. 6 - Prob. 59IPCh. 6 - Prob. 60IPCh. 6 - Prob. 61IPCh. 6 - Prob. 62CPCh. 6 - Prob. 64CP
Knowledge Booster
Similar questions
- A problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forwardThe compound eutypine is an antibacterial agent isolated from the fungus Eutypa lata. This fungus results in a disease common to vineyards called eutyposis. Give a sequence of reactions that will take the following reactant and give eutypine when the other reactants used in the sequence are acetylene and acetone.arrow_forwardAcid-catalyzed bromination of pentan-2-one (CH3COCH2CH2CH3) forms two products: BrCH2COCH2CH2CH3 (A)and CH3COCH(Br)CH2CH3 (B). Explain why the major product is B, with the Br atom on the more substituted side of the carbonyl group.arrow_forward
- Heterocyclic compounds plays an important role in our daily life. They are mainly used in pharmaceutical and agrochemical products to name a few. 3. It is required to introduce a halogen group to a five membered ring, thiophene. Discuss the reaction mechanism involved in the reaction by selecting a suitable halogen group and analyze why a particular substituted product obtained after the reaction is predominant over the other possible product(s) with the help of reactions.arrow_forwardWhich of the presented structures (A-F) is the expected product of the following Friedel-Craft alkylation reaction?arrow_forwardWhen the compound shown below undergoes acid-catalyzed dehydration, a ring expansionoccurs to give the fused-ring product. What type of intermediate is formed in this reaction?Explain the ring expansion in terms of the reaction mechanismarrow_forward
- When the following compound is hydrated in the presence of acid, the unreacted alkene is found to have retained the deuterium atoms. What does this tell you about the mechanism for hydration?arrow_forwardTerreic acid, shown below, is a naturally occurring antibiotic metabolite of the fungus Aspergillus terreus. Terreic acid hinders bacterial growth by covalently binding to (and thereby deactivating) the biosynthetic enzyme MurA, which is responsible for synthesizing the bacterial cell wall. In aqueous environments, terreic acid tautomerizes to a more stable enol form. Draw the two most stable enol tautomers of terric acid. Circle the tautomer above that you would expect to predominate inside a cellular environment.arrow_forwardProvide an arrow-pushing mechanism for the following reaction. There may be other major products formed in the reaction, but you should only show the mechanism to get to the product shown. Ignore stereochemistry.arrow_forward
- Consider the following chemical transformation:The transformation takes place via twosequential pericyclic reactions. Identify the two reactions and give a critical explanationwhether the reactions are allowable.arrow_forwardWhen toluene is treated with bromine (shown), the bromine doesn't react with the double bond to form a viscinal dihalide, but instead a substitution of one of the benzylic hydrogens takes place. Why?arrow_forwardDraw the mechanism of the photochemistry reaction of benzophenone when reacted with isopropyl alcohol, H+, and UV light to form the product benzopinacol. Show arrows for electron movement for every step and draw in line structurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning