
Concept explainers
(a)
Interpretation: The compound (1) converts to compound (5) through the mechanistic sequence under basic conditions and in the presence of
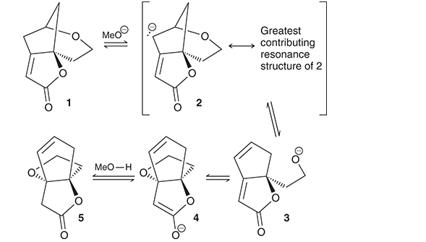
Concept introduction: Resonance is the phenomenon of the interaction of lone pairs of electrons and pi electrons in a molecule. More resonance contributes to more stability of the molecule. The overall structure can be drawn as the sum of all resonating structures which is called a resonance hybrid.
(b)
Interpretation: The compound (1) converts to compound (5) through the mechanistic sequence under basic conditions and in the presence of
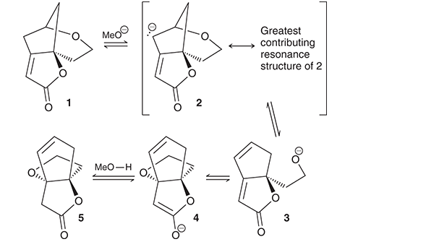
Concept introduction: The chemical transformation can be shown by the arrow pushing method in which the curved arrows show the movement of electrons or protons. In a
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- e which of the following benzene compounds will be more reactive than ben vill be less reactive than benzene. (1 point) NO2 NH3 NH2arrow_forward3. The following reaction is discussed in a later chapter of the textbook. It consists of four mechanistic steps. For each step (A—D), a. Draw in all implied lone pairs. b. Draw the appropriate curved arrows to show the bonds formation and bond breaking that occur. A В C Н Н D CH3 нон Н он - обна ΘΗ + Н CH3 CH3 собом - стран ΘῊ + H Hн -CH3 + н CH3 0. НН Н - н-о-н H нн + CH3 CH3 О-Н CH3 но нarrow_forwardExplain me why #3 is the ring flip for the compound shownarrow_forward
- 1. Draw ethylbenzene and put a + on the carbon next to the benzene (innermost carbon in ethyl group). Show all resonance forms for this ion. 2. draw the naphthalene and put one - on the neighboring carbon of the "crossroads". show every resonance forms for this ion. 3. Draw benzoic acid and draw on all p-orbitals.arrow_forwardAdd curved arrows to explain the following intramolecular rearrangement. What are the numeric codes for your arrows? Numbers in red = bonds Numbers in green = atoms A B C D 2 4 ...O -O.I 84, 32 CH3 H 82, 36 65, 48 84, 56 3 I O -H 7 CH3 OH :O: Oarrow_forward2. Draw curved arrows that show the bond changes for each chemical step shown below. CH3 :: H3C e + H3C a) b) C X CH3 K+ :OH H3C CH3 + KI T: + H₂Oarrow_forward
- Explain why the following reaction takes place as shown. Be sure to fully explain why the reaction sites are used and what is happening to the formal charges. Include a possible explanation for the negatively charged oxygen reacting with the carbon bonded to the CI rather than the carbon bonded to the SH. SH -- د SHarrow_forwardDraw the molecular orbital diagram of a linear C-H (i.e. one carbon atom bonded to one hydrogen atom). Draw out the MOs for orbitals containing electrons. 1. Would you expect C-H to behave as a carbocation, carbanion, or radical? 2. Would removal of one electron from C-H to generate C-H+ increase or decrease the bond length between carbon and hydrogen?arrow_forward4. The following reaction is discussed in a later chapter of the textbook. It consists of four mechanistic steps. For each step (1-3), a. Draw in all implied lone pairs. b. Draw the appropriate curved arrows to show the bonds formation and bond breaking that occur. 1 2 H 3 + + H قرة - . . ق нон H H + H H H ... . قرق - ة . -قرة H H 1 + O H H H H Harrow_forward
- For each compound below, draw in all missing lone pairs and indicate whether each lone pair is delocalized or localized. Then, use that information to determine the hybridization state for each atom that has a lone pair. Hint: allylic lone pairs are delocalized and do not count towards the steric number. H2N. HO (a). (b).arrow_forward4. Which of the following pairs have a higher boiling point? Under each one, list the most dominant IMF (strongest) present. a) LiCl or HCI b) c) d) e) NH3 Xe NO or or or CH3CH2CH3 or PH3 12 N₂ CH3CH2OHarrow_forwardBased on the direction of twist of the following groups, assign an R or S orientation. H HO .C CHS NH I Br P b. a. [Select] [Select] C. [Select] > CI b. OH L Br CH C. B3arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
