
Concept explainers
a.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangement of atoms in the molecule are said to be isomers of each other.
a.
Answer to Problem 32PP
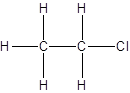
Explanation of Solution
For the first isomer structure, all two-carbon atoms are written in a straight chain and the chlorine atom is bonded to one of the carbon atoms as:
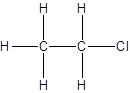
There is no other possibility for the different structural formula
b.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangement of atoms in the molecule are said to be isomers of each other.
b.
Answer to Problem 32PP
Isomer I:
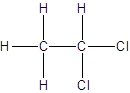
Isomer II:
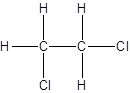
Explanation of Solution
The different structural the arrangement of atoms in the molecule
- All two-carbon atoms are written in a straight chain and two chlorine atoms are bonded to one of the carbon atoms resulting in:
- Interchanging the position of one hydrogen atom at first carbon with one chlorine atom of the second carbon atom.
Isomer I:
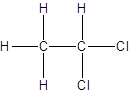
Isomer II:
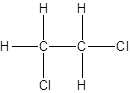
c.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangements of atoms in the molecule are said to be isomers of each other.
c.
Answer to Problem 32PP
Isomer I:
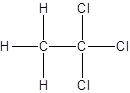
Isomer II:
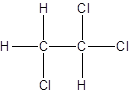
Explanation of Solution
The different structural arrangement of atoms in the molecule
- All two-carbon atoms are written in a straight chain and three chlorine atoms are bonded to one of the carbon atoms resulting in:
- Interchanging the position of one hydrogen atom at first carbon with one chlorine atom of the second carbon atom.
Isomer I:
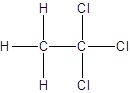
Isomer II:
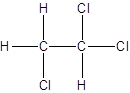
d.
Interpretation: All the constitutional isomers that have molecular formulas
Concept Introduction: The molecules that possess the same molecular formula but differ in the structural arrangements of atoms in the molecule are said to be isomers of each other.
d.
Answer to Problem 32PP
Isomer I:
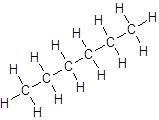
Isomer II:
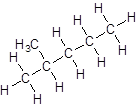
Isomer III:
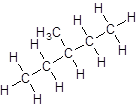
Isomer IV:
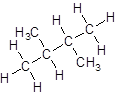
Isomer V:
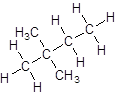
Explanation of Solution
The different structural arrangement of atoms in the molecule
- All six-carbon atoms are written in a straight chain resulting in:
- Five-carbon atoms are written in a straight chain and a methyl group is attached to the second carbon resulting in:
- Five-carbon atoms are written in a straight chain and a methyl group is attached to the third carbon resulting in:
- Four-carbon atoms are written in a straight chain and a methyl group is attached to the second and third carbon resulting in:
- Four-carbon atoms are written in a straight chain and two methyl groups are attached to the second carbon resulting in:
Isomer I:
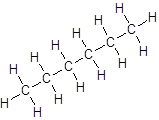
Isomer II:
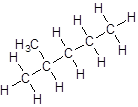
Isomer III:
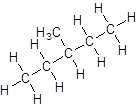
Isomer IV:
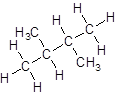
Isomer V:
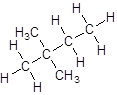
Want to see more full solutions like this?
Chapter 1 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- 3arrow_forwardWhat is the chemical formula for the molecule represented by the skeletal structure below? = Select one: a. Cannot be determined from this skeletal structure b. C2 c. C2H2 d. CH4arrow_forward8. CH3CCH (propyne or methylacetylene) Projection Drawing: Perspective Drawing: lives a. Predict the angel HCC (the first carbon) CCC. b. Predict the molecular geometry around each carbon atomarrow_forward
- Which of the following C-C bonds is the shortest? A. в D E Select one: O a. A O b. B O c. C O d. D О е. Еarrow_forwardWhich of the following bonds is least polar? Select one: а. С-Н b. B-H C. Al-H d. N-H e. P-Harrow_forwardWhich is the correct representation of formal charge of the Leasing th H-C-N H:6: A. C. H- H H-C H B. D. H-C-N Harrow_forward
- D. @ 2 39 F2 Draw charge minimized Lewis structures for the following compounds: W a. HCN S a. What types of intermolecular forces would you predict for each of the compounds above? b. # 3 X command 80 F3 E D $ 4 C 000 888 FA R APR 26 F % ar 20 5 FS T G ^ 6 F6 Y V B H & 7 44 F7 U N * + m 8 J b. IOF I DII FO - M shapes and physical ( 9 K DD F9 0 < I 0 L command F10 P . J - : ; I 4 option F11 { + " [ ? ~~ = 1 I 40 4 112 } ] delete 22 1 1 1 return shiftarrow_forward2. What type of chemical bonding is present in the following compound? CH3CH2CH2CH2Br O a. A O b. B O C. C O d. D A) Polar covalent B) Hydrogen C) lonic D) polar covalent and non-polar covalentarrow_forwardClassify each bond in the following molecules as σ or л: a. CH3 H b. CH3-C=N C. H& OCH3arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
