
Concept explainers
a)
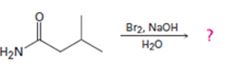
Interpretation:
The product of the reaction given is to be predicted and a mechanism for the reaction is to be provided.
Concept introduction:
In Hofmann degradation reaction amides give
b)
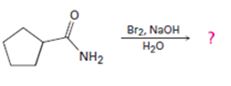
Interpretation:
The product of the reaction given is to be predicted and a mechanism for the reaction is to be provided.
Concept introduction:
In Hofmann degradation reaction amides give amines with one carbon less than that in amides. Amides react in the presence of a base to give an isocyanate which upon hydrolysis yields the amine liberating CO2.
c)
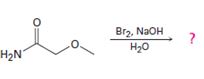
Interpretation:
The product of the reaction given is to be predicted and a mechanism for the reaction is to be provided.
Concept introduction:
In Hofmann degradation reaction amides give amines with one carbon less than that in amides. Amides react in the presence of a base to give an isocyanate which upon hydrolysis yields the amine liberating CO2.
d)
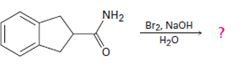
Interpretation:
The product of the reaction given is to be predicted and a mechanism for the reaction is to be provided.
Concept introduction:
In Hofmann degradation reaction amides give amines with one carbon less than that in amides. Amides react in the presence of a base to give an isocyanate which upon hydrolysis yields the amine liberating CO2.
Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- The Ksp for lead iodide ( Pbl₂) is 1.4 × 10-8. Calculate the solubility of lead iodide in each of the following. a. water Solubility = mol/L b. 0.17 M Pb(NO3)2 Solubility = c. 0.017 M NaI mol/L Solubility = mol/Larrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forward
- Only 100% sure experts solve it correct complete solutions need to get full marks it's my quiz okkkk.take your time but solve full accurate okkk chemistry expert solve itarrow_forwardPleasssssseeee solve this question in cheeemsirty, thankss sirarrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardPart A Give the IUPAC name and a common name for the following ether: CH3-CH2-O-CH2-CH2-CH3 Spell out the full names of the compound in the indicated order separated by a comma. Submit My Answers Give Up Part B Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma. Submit My Answers Give Uparrow_forwardFrenkel and Schottky are intrinsic or extrinsic defects, point or linear defects.arrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


