
a)
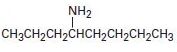
Interpretation:
To explain the Hofmann elimination of the given
Concept introduction:
The Hofmann elimination is an E2 reaction that converts an amine into an
b)
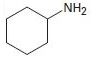
Interpretation:
To explain the Hofmann elimination of the given amines.
Concept introduction:
Just like alcohol, amines can be converted into alkenes by an elimination reaction.
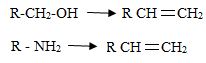
But because an amide ion, -NH2 is a poor leaving group, it must be converted into a better leaving group.
c)
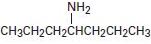
Interpretation:
To explain the Hofmann elimination of the given amines.
Concept introduction:
The given amine has two symmetrical groups is CH3CH2CH2 – attached to the carbon C-4- carrying the amine –NH2 group.
d)
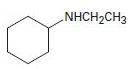
Interpretation:
To explain the Hofmann elimination of the given amines.
Concept introduction:
The Hofmann elimination is an E2 elimination that converts an amine to an alkene and occurs with non-Zaitsev regiochemistry to form the less highly substituted double bond alkene as the major product.
Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- Give the products expected when the following tertiary amines are treated with a peroxyacid and heated.(a) N,N-dimethylhexan-2-aminearrow_forwardGive the products expected when the following tertiary amines are treated with a peroxyacid and heated.N-ethylpiperidinearrow_forwardShow how Gabriel syntheses are used to prepare the following amines. g@aminobutyric acidarrow_forward
- Account for the following :(i) Primary amines (R-NH2) have higher boiling point than tertiary amines (R3N).(ii) Aniline does not undergo Friedel – Crafts reaction.(iii) (CH3)2NH is more basic than (CH3)3N in an aqueous solution.arrow_forwardRank the following compounds in order of increasing basicity: I. p-nitroaniline III. N-methylaniline II. p-aminobenzaldehyde IV. p-methylanilinearrow_forwardShow how Gabriel syntheses are used to prepare the following amines. ) hexan-1-aminearrow_forward
- (1) Which is the most basic amine and which is a secondary amine? (2) Which can undergo hydrolysis? (3) Which gives a diazonium salt upon reaction with HNO2, HCl at 0oC?arrow_forwardWhat products are obtained when the following tertiary amines react with hydrogen peroxide followed by heat?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

